



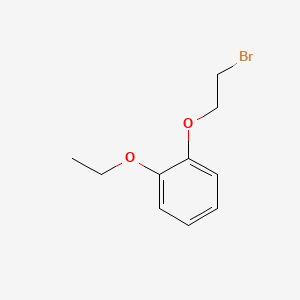

1. 2-(2-ethoxyphenoxy)ethyl Bromide
2. 3259-03-8
3. 2-(2-ethoxyphenoxy)ethylbromide
4. Benzene, 1-(2-bromoethoxy)-2-ethoxy-
5. 1-(2-bromoethoxy)-2-ethyoxybenzene
6. Mfcd02030483
7. Gmj3274102
8. 2-(2-bromoethoxy)phenetole
9. 1-(2-bromoethoxy)-2-ethoxy-benzene
10. Tamsulosin Impurity I
11. Ethyl4-boronocinnamate
12. Schembl1412326
13. 2-(o-ethoxyphenoxy)ethylbromide
14. Unii-gmj3274102
15. 2-(o-ethoxyphenoxy)ethyl Bromide
16. Dtxsid10186283
17. Amy31226
18. Zinc2388622
19. Akos000164018
20. Ac-5615
21. Cs-w002871
22. Ds-1212
23. Sy030339
24. B3191
25. Ft-0634750
26. En300-93023
27. 259e038
28. Q27279176
29. Tamsulosin Hydrochloride Impurity I [ep Impurity]
30. 1-(2-bromoethoxy)-2-ethoxybenzene;2-(2-bromoethoxy)phenetole
| Molecular Weight | 245.11 g/mol |
|---|---|
| Molecular Formula | C10H13BrO2 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 244.00989 g/mol |
| Monoisotopic Mass | 244.00989 g/mol |
| Topological Polar Surface Area | 18.5 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




