



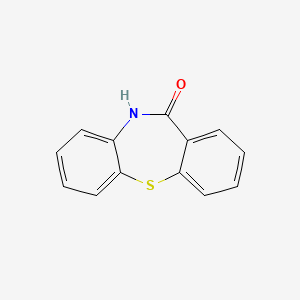

1. 10,11-dihydro-11-oxodibenzo[b,f][1,4]thiazepine
2. 3159-07-7
3. 5h-benzo[b][1,4]benzothiazepin-6-one
4. Dibenzo[b,f][1,4]thiazepine-11-[10h]one
5. Dibenzothiazepinone
6. Nsc653252
7. Fl6fg1e3wf
8. Gnf-pf-769
9. 10h-dibenzo[b,f][1,4]thiazepin-11-one
10. Mfcd00901197
11. Nsc-653252
12. Dibenzo(b,f)(1,4)thiazepin-11(10h)-one
13. Dibenzo[b,f][1,4]thiazepine-11(10-h)-one
14. Dibenzo[b,f][1,4]thiazepine-11-(10h)one
15. Dibenzo[b,f][1,4]thiazepinone
16. 10,11-dihydro-11-oxodibenz[b,f][1,4]thiazepine
17. Dibenzo(b,f)(1,4)thiazepine-11(10-h)-one
18. 10,11-dihydro-11-oxodibenz(b,f)(1,4)thiazepine
19. 10,11-dihydro-11-oxodibenzo(b,f)(1,4)thiazepine
20. Quetiapine Impurity G
21. Quetiapine Impurtiy G
22. Dibenzo[b,4]thiazepinone
23. Unii-fl6fg1e3wf
24. Dibenzothiazepinone [usp]
25. Schembl8988
26. Mls000698398
27. Quetiapine Related Compound G
28. Chembl598054
29. Zinc14023
30. Dtxsid10953553
31. Hms2666m13
32. {dibenzo[b,f][1,4]thiazepinone}
33. Bcp30999
34. Cs-m2280
35. Quetiapine Fumarate Impurity G [ep]
36. Quetiapine Related Compound G [usp]
37. Dibenzo[b,f][1,4]thiazepin-11-ol
38. Akos005216133
39. Ac-5341
40. Dibenzo-[b,f][1,4]thiazepin-11-one
41. Ds-0806
42. Fs-3018
43. Sb67310
44. Dibenzo-[b,f] [1,4]thiazepin-11-one
45. Dibenzothiazepinone [usp Impurity]
46. Smr000224931
47. Sy047017
48. 10h-dibenzo[b,f][1,4]thiazepine-11-one
49. Db-005658
50. Dibenz[b,f][1,4]thiazepin-11(10h)-one
51. Am20040155
52. D3693
53. Dibenzo[b,f][1,4]thiazepine-11(10-h)one
54. Ft-0600897
55. Di-benzo[b,f ][1,4]thiazepin-11(10h)-one
56. Dibenzo[b,f][1,4]thiazepin-11(10h)-one #
57. O10365
58. Quetiapine Related Compound G [usp-rs]
59. 5,6-dihydro-6-oxodibenzo(b,f)-1,4-thiazepine
60. Dibenzo [b-f] [1-4]thiazepine 11-[10h]one
61. 159d077
62. Ae-641/30103015
63. 10,11-dihydro-11-oxodibenz[b,f] [1,4]thiazepine
64. 10,11-dihydrodibenzo[b,f][1,4] Thiazepin-11-one
65. 10,11-dihydrodibenzo[b,f][1,4]thiazepin-11-one
66. Q-200955
67. Quetiapine Fumarate Impurity G [ep Impurity]
68. Quetiapine Related Compound G [usp Impurity]
69. 1-(2,3-dimethyl-1h-indol-6-yl)-n,n-dimethylmethanamine
70. Quetiapine Impurtiy G (dibenzo[b,f][1,4]thiazepine-11(10-h)-one)
71. 2-thia-9-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-10-one
72. 10,11-dihydro-11-oxodibenzo[b,f][1,4]thiazepine Pound>>dibenzo[b,f][1,4]thiazepin-11(10h)-one
| Molecular Weight | 227.28 g/mol |
|---|---|
| Molecular Formula | C13H9NOS |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 227.04048508 g/mol |
| Monoisotopic Mass | 227.04048508 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 281 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





