




1. 2689-39-6
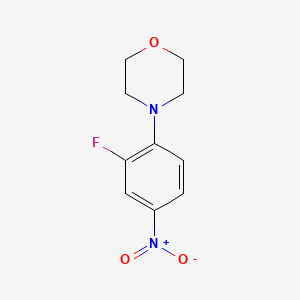
2. 4-(2-fluoro-4-nitro-phenyl)-morpholine
3. Morpholine, 4-(2-fluoro-4-nitrophenyl)-
4. 3-fluoro-4-morpholinonitrobenzene
5. Mfcd03138381
6. Cambridge Id 6614516
7. Schembl210786
8. 3fluoro-4-morpholinonitrobenzene
9. Dtxsid60387723
10. Zeqcfssbzpzefj-uhfffaoysa-n
11. 36r3kp66k8
12. 3-fluoro-4-morpholinyl Nitrobenzene
13. 3-fluoro-4-morpholinyl-nitrobenzene
14. Stk351957
15. 1-fluoro-2-morpholino-5-nitrobenzene
16. 1-morpholino-2-fluoro-4-nitrobenzene
17. Akos005168040
18. Akos015922545
19. N-(2-fluoro-4-nitrophenyl)morpholine
20. Am86816
21. Sy005339
22. A818663
23. Sr-01000238859
24. Sr-01000238859-1
| Molecular Weight | 226.20 g/mol |
|---|---|
| Molecular Formula | C10H11FN2O3 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 58.3 |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




