




1. 22009-38-7
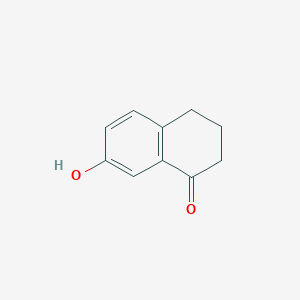
2. 7-hydroxy-3,4-dihydronaphthalen-1(2h)-one
3. 7-hydroxy-3,4-dihydro-2h-naphthalen-1-one
4. 7-hydroxy-3,4-dihydro-1(2h)-naphthalenone
5. 1(2h)-naphthalenone, 3,4-dihydro-7-hydroxy-
6. Mfcd01312225
7. 7-hydroxytetralin-1-one
8. 7-hydroxytetralone
9. 7-hydroxy-tetralone
10. 7-hydroxy-1-tetralone (5)
11. Schembl385554
12. Dtxsid00341263
13. Bdbm166645
14. Amy14181
15. Bcp16590
16. Zinc24627693
17. Akos006229303
18. Cs-w022544
19. Mb01768
20. Ac-27437
21. As-10078
22. Sy045775
23. 3,4-dihydro-7-hydroxy-1(2h)-naphthalenone
24. Bb 0257319
25. Ft-0648907
26. H1650
27. 7-hydroxy-3,4-dihydro-1(2h)-naphthalenone #
28. En300-191143
29. A851740
30. 7-hydroxy-1,2,3,4-tetrahydronaphthalen-1-one
31. Z1198156579
| Molecular Weight | 162.18 g/mol |
|---|---|
| Molecular Formula | C10H10O2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 37.3 |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 188 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


