



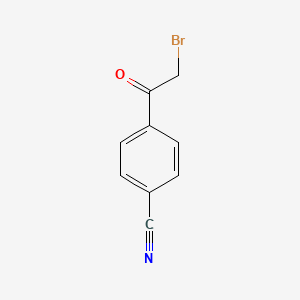

1. 20099-89-2
2. 4-cyanophenacyl Bromide
3. 4-(bromoacetyl)benzonitrile
4. 2-bromo-4'-cyanoacetophenone
5. Benzonitrile, 4-(bromoacetyl)-
6. P-cyanophenacyl Bromide
7. 4-(2-bromo-acetyl)-benzonitrile
8. E6t2np32gc
9. 2-bromo-4'-cyano Acetophenone
10. Chembl1801622
11. Benzonitrile, 4-(2-bromoacetyl)-
12. Nsc-157569
13. 4-cyanophenacyl Bromide 97%
14. Mfcd00052931
15. Nsc 157569
16. P-bromoacetylbenzonitrile
17. 4-bromoacetylbenzonitrile
18. 4-cyano Phenacyl Bromide
19. 4-bromoacetyl Benzonitrile
20. 4-bromoacetyl-benzonitrile
21. Unii-e6t2np32gc
22. Schembl250688
23. A-bromo-4'-cyano-acetophenone
24. 4-(bromoacetyl)benzonitrile #
25. 2-bromo-4'-cyano-acetophenone
26. 4-(2-bromoacetyl)-benzonitrile
27. The Intermideate Of Isavuconazole
28. Dtxsid00173920
29. Am835
30. Zinc166064
31. 2-bromo-1-(4-cyanophenyl)ethanone
32. Act00938
33. Bcp10300
34. Cs-m3110
35. Str07969
36. Bdbm50347519
37. Ck2151
38. Nsc157569
39. Stk279014
40. 2-bromo-1-(4-cyanophenyl)-ethanone
41. 2-bromo-4'-cyanoacetophenone, 96%
42. 2-bromo-4-cyano Acetophenone
43. 4-(2-bromoacetyl)benzonitrile-[d4]
44. Akos000117765
45. Upcmld0enat5695579:001
46. Ac-31070
47. Bp-13004
48. Db-045087
49. Ft-0611444
50. En300-13898
51. J-013010
52. 2-bromo-4 Inverted Exclamation Marka-cyanoacetophenone
53. F0001-0711
| Molecular Weight | 224.05 g/mol |
|---|---|
| Molecular Formula | C9H6BrNO |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 222.96328 g/mol |
| Monoisotopic Mass | 222.96328 g/mol |
| Topological Polar Surface Area | 40.9 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 210 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





















