




1. 165115-73-1
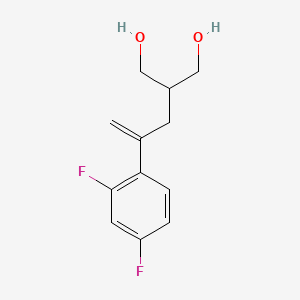
2. 2-(2-(2,4-difluorophenyl)allyl)propane-1,3-diol
3. 1,3-propanediol, 2-[2-(2,4-difluorophenyl)-2-propen-1-yl]-
4. 2-[2-(2,4-difluorophenyl)prop-2-enyl]propane-1,3-diol
5. 2-2-(2,4-difluoro-phenyl)-allyl-propane-1,3-diol
6. 2-[2-(2,4-difluorophenyl)prop-2-en-1-yl]propane-1,3-diol
7. 2-(2-(2,4-difluorophenyl)prop-2-en-1-yl)propane-1,3-diol
8. Posaconazole Intermediate-7
9. Ec 605-390-1
10. Schembl12186064
11. Dtxsid30453909
12. 1,3-propanediol, 2-[2-(2,4-difluorophenyl)-2-propenyl]-
13. Amy14819
14. Bcp13096
15. Zinc34623871
16. Akos015912312
17. Ac-8490
18. Sb34124
19. As-75728
20. D92910
21. 115p731
22. 2-[2-(2,4-difluoro-phenyl)-allyl]-propane-1,3-diol
23. 2-[2-(2,4-difluorophenyl)-2-propenyl]-1,3-propanediol
| Molecular Weight | 228.23 g/mol |
|---|---|
| Molecular Formula | C12H14F2O2 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 228.09618601 g/mol |
| Monoisotopic Mass | 228.09618601 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 229 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



