

X



1. 1643330-62-4
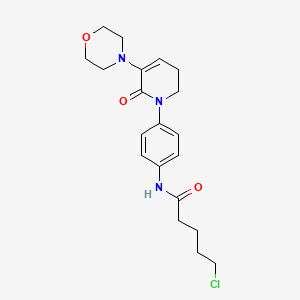
2. 5-chloro-n-(4-(5-morpholino-6-oxo-3,6-dihydropyridin-1(2h)-yl)phenyl)pentanamide
3. V9yk56w8pq
4. 5-chloro-n-(4-(5,6-dihydro-3-(4-morpholinyl)-2-oxo-1(2h)-pyridinyl)phenyl)pentanamide
5. Pentanamide, 5-chloro-n-(4-(5,6-dihydro-3-(4-morpholinyl)-2-oxo-1(2h)-pyridinyl)phenyl)-
6. Unii-v9yk56w8pq
7. Schembl16730279
8. Cs-m2421
9. F19392
| Molecular Weight | 391.9 g/mol |
|---|---|
| Molecular Formula | C20H26ClN3O3 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 391.1662694 g/mol |
| Monoisotopic Mass | 391.1662694 g/mol |
| Topological Polar Surface Area | 61.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 541 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




