




1. 147403-65-4
2. Schembl16367902
3. Amy4361
4. Dtxsid10570360
5. Mfcd21496678
6. Akos016004226
7. Ds-3760
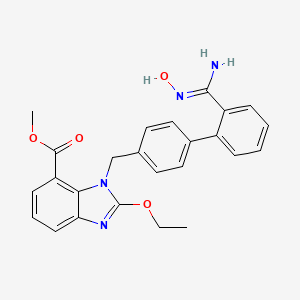
8. 1h-benzimidazole-7-carboxylic Acid, 2-ethoxy-1-[[2'-[(hydroxyamino)iminomethyl][1,1'-biphenyl]-4-yl]methyl]-, Methyl Ester
9. Ac-25906
10. (z)-methyl 2-ethoxy-3-((2-(n-hydroxycarbamimidoyl)biphenyl-4-yl)methyl)-3h-benzo[d] Imidazole-4-carboxylate
11. Methyl 2-ethoxy-1-{[2'-(n'-hydroxycarbamimidoyl)[1,1'-biphenyl]-4-yl]methyl}-1h-benzimidazole-7-carboxylate
12. Methyl 2-ethoxy-3-[[4-[2-[(z)-n\'-hydroxycarbamimidoyl]phenyl]phenyl]methyl]benzimidazole-4-carboxylate
| Molecular Weight | 444.5 g/mol |
|---|---|
| Molecular Formula | C25H24N4O4 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 444.17975526 g/mol |
| Monoisotopic Mass | 444.17975526 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 681 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




