



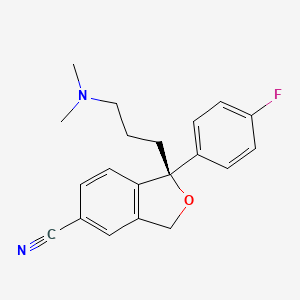

1. Escitalopram Oxalate
2. Lexapro
1. 128196-01-0
2. (s)-citalopram
3. S(+)-citalopram
4. (+)-citalopram
5. Seroplex
6. S-(+)-citalopram
7. Esertia
8. Cipralex
9. Escitalopram [inn]
10. Esitol
11. (1s)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-2-benzofuran-5-carbonitrile
12. Escitalopram (inn)
13. Chembl1508
14. (s)-1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
15. 4o4s742any
16. Chebi:36791
17. (+)-(s)-1-(3-(dimethylamino)propyl)-1-(p-fluorophenyl)-5-phthalancarbonitrile
18. (1s)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3h-2-benzofuran-5-carbonitrile
19. Lu-26-054
20. 5-isobenzofurancarbonitrile, 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-, (1s)-
21. 5-isobenzofurancarbonitrile, 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydro-, (1s)-
22. Esertia (tn)
23. Escitalopram [inn:ban]
24. Escitalopramum
25. Unii-4o4s742any
26. 68p
27. Spectrum_001401
28. Tocris-1427
29. Spectrum2_000551
30. Spectrum3_001062
31. Spectrum4_001212
32. Spectrum5_001693
33. Escitalopram [mi]
34. Lopac-c-7861
35. Escitalopram [vandf]
36. Bidd:pxr0135
37. Schembl34948
38. Bspbio_002644
39. Kbiogr_001644
40. Kbioss_001881
41. Escitalopram [who-dd]
42. Spbio_000621
43. Gtpl7177
44. Dtxsid8048440
45. Hsdb 8410
46. Kbio2_001881
47. Kbio2_004449
48. Kbio2_007017
49. Kbio3_001864
50. Hms2089o08
51. Escitalopram [ep Monograph]
52. (s)-citalopram;s-(+)-citalopram
53. Bcp12154
54. Zinc3800706
55. Bdbm50302225
56. Akos017343470
57. 2-methoxyethylp-toluenesulfonate
58. Ac-1594
59. Ac-4508
60. Cs-2053
61. Db01175
62. Sb17453
63. (1r)-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-3h-isobenzofuran-5-carbonitrile
64. (1s)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3h-isobenzofuran-5-carbonitrile
65. Mrf-0000226
66. Ncgc00015267-01
67. Ncgc00015267-02
68. Ncgc00015267-03
69. Ncgc00015267-06
70. Ncgc00025160-01
71. Ncgc00178555-01
72. Ncgc00178555-07
73. Hy-14258
74. B1183
75. D07913
76. Ab00698374-07
77. Ab00698374-09
78. Ab00698374_10
79. A805793
80. Q423757
81. J-005570
82. Brd-k70301876-034-02-0
83. (s)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5 Carbonitrile
84. S-(+)-1-(3-(dimethylamino)propyl)-1-(p-fluorophenyl)-5-phthalancarbonitrile
85. (s)-(+)-1-[3-(dimethylamino) Propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofuran Carbonitrile
86. (s)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofuran Carbonitrile
87. S-(+)-5-isobenzofurancarbonitrile, 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydro-
| Molecular Weight | 324.4 g/mol |
|---|---|
| Molecular Formula | C20H21FN2O |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 36.3 |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Serotonin Uptake Inhibitors; Antidepressive Agents, Second-Generation
National Library of Medicine's Medical Subject Headings. Escitalopram. Online file (MeSH, 2018). Available from, as of March 7, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Escitalopram is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 7, 2018: https://clinicaltrials.gov/
Lexapro (escitalopram) is indicated for the acute and maintenance treatment of major depressive disorder in adults and in adolescents 12 to 17 years of age. A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least five of the following nine symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt or suicidal ideation. /Included in US product label/
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
Lexapro is indicated for the acute treatment of Generalized Anxiety Disorder (GAD) in adult. Generalized Anxiety Disorder (DSM-IV) is characterized by excessive anxiety and worry (apprehensive expectation) that is persistent for at least 6 months and which the person finds difficult to control. It must be associated with at least 3 of the following symptoms: restlessness or feeling keyed up or on edge, being easily fatigued, difficulty concentrating or mind going blank, irritability, muscle tension, and sleep disturbance. /Included in US product label/
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
For more Therapeutic Uses (Complete) data for Escitalopram (9 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Lexapro or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Lexapro is not approved for use in pediatric patients less than 12 years of age.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
Potentially life-threatening serotonin syndrome or neuroleptic malignant syndrome (NMS)-like reactions have been reported with selective serotonin-reuptake inhibitors (SSRIs), including escitalopram, and selective serotonin- and norepinephrine-reuptake inhibitors (SNRIs) alone, but particularly with concurrent use of other serotonergic drugs (including serotonin (5-hydroxytryptamine; 5-HT) type 1 receptor agonists ("triptans")), drugs that impair the metabolism of serotonin (e.g., MAO inhibitors), or antipsychotics or other dopamine antagonists. Manifestations of serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or GI symptoms (e.g., nausea, vomiting, diarrhea). In its most severe form, serotonin syndrome may resemble NMS, which is characterized by hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation in vital signs, and mental status changes. Patients receiving escitalopram should be monitored for the development of serotonin syndrome or NMS-like signs and symptoms.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2462
The use of monoamine oxidase inhibitors (MAOIs) intended to treat psychiatric disorders with Lexapro or within 14 days of stopping treatment with Lexapro is contraindicated because of an increased risk of serotonin syndrome. The use of Lexapro within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated. Starting Lexapro in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
If concomitant use of Lexapro with other serotonergic drugs including, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan, amphetamine and St. John's Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
For more Drug Warnings (Complete) data for Escitalopram (20 total), please visit the HSDB record page.
Escitalopram is indicated for both acute and maintenance treatment of major depressive disorder (MDD) and for the acute treatment of generalized anxiety disorder (GAD). It is additionally indicated for symptomatic relief of obsessive-compulsive disorder (OCD) in Canada.
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
N06AB10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AB - Selective serotonin reuptake inhibitors
N06AB10 - Escitalopram
Absorption
Absorption of escitalopram following oral administration is expected to be almost complete, with an estimated absolute bioavailability of approximately 80%. Tmax occurs after about 4-5 hours. Cmax and AUC appear to follow dose proportionality - at steady state, patients receiving 10mg of escitalopram daily had a Cmax of 21 ng/mL and a 24h AUC of approximately 360 ng*h/mL, while patients receiving 30mg daily had a roughly 3-fold increase in both Cmax and 24h AUC, comparatively.
Route of Elimination
After oral administration of escitalopram, approximately 8% of the total dose is eliminated in the urine as unchanged escitalopram and 10% is eliminated in the urine as S-desmethylcitalopram. The apparent hepatic clearance of escitalopram amounts to approximately 90% of the total dose.
Volume of Distribution
Escitalopram appears to distribute extensively into tissues, with an apparent volume of distribution of approximately 12-26 L/kg.
Clearance
The oral plasma clearance of escitalopram is 600 mL/min, of which approximately 7% is due to renal clearance.
/MILK/ Escitalopram is excreted in human breast milk. Limited data from women taking 10-20 mg escitalopram showed that exclusively breast-fed infants receive approximately 3.9% of the maternal weight-adjusted dose of escitalopram and 1.7% of the maternal weight-adjusted dose of desmethylcitalopram.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
The absolute bioavailability of citalopram is about 80% relative to an intravenous dose, and the volume of distribution of citalopram is about 12 L/kg. Data specific on escitalopram are unavailable. The binding of escitalopram to human plasma proteins is approximately 56%.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
Following a single oral dose (20 mg tablet or solution) of escitalopram, peak blood levels occur at about 5 hours. Absorption of escitalopram is not affected by food.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
The metabolism of escitalopram is mainly hepatic, mediated primarily by CYP2C19 and CYP3A4 and, to a lesser extent, CYP2D6. Oxidative N-demethylation by the CYP enzyme system results in S-desmethylcitalopram (S-DCT) and S-didesmethylcitalopram (S-DDCT) - these metabolites do not contribute to the pharmacologic activity of escitalopram, and exist in the plasma in small quantities relative to the parent compound (28-31% and <5%, respectively). There is also some evidence that escitalopram is metabolized to a propionic acid metabolite by monoamine oxidase A and B in the brain, and that these enzymes constitute the major route of escitalopram metabolism in the brain.
The antidepressant escitalopram is predominantly metabolized by the polymorphic CYP2C19 enzyme. The authors investigated the effect of CYP2C19 genotype on exposure and therapeutic failure of escitalopram in a large patient population. A total of 4,228 escitalopram serum concentration measurements from 2,087 CYP2C19-genotyped patients 10-30 hours after drug intake were collected retrospectively from the drug monitoring database at Diakonhjemmet Hospital in Oslo. The patients were divided into subgroups based on CYP2C19 genotype: those carrying inactive (CYP2C19Null) and gain-of-function (CYP2C19*17) variant alleles. The between-subgroup differences in escitalopram exposure (endpoint: dose-harmonized serum concentration) and therapeutic failure (endpoint: switching to another antidepressant within 1 year after the last escitalopram measurement) were evaluated by multivariate mixed model and chi-square analysis, respectively. Compared with the CYP2C19*1/*1 group, escitalopram serum concentrations were significantly increased 3.3-fold in the CYP2C19Null/Null group, 1.6-fold in the CYP2C19*Null/*1 group, and 1.4-fold in the CYP2C19Null/*17 group, whereas escitalopram serum concentrations were significantly decreased by 10% in the CYP2C19*1/*17 group and 20% in the CYP1C19*17/*17 group. In comparison to the CYP2C19*1/*1 group, switches from escitalopram to another antidepressant within 1 year were 3.3, 1.6, and 3.0 times more frequent among the CYP2C19Null/Null, CYP2C19*1/*17, and CYP1C19*17/*17 groups, respectively. The CYP2C19 genotype had a substantial impact on exposure and therapeutic failure of escitalopram, as measured by switching of antidepressant therapy. The results support the potential clinical utility of CYP2C19 genotyping for individualization of escitalopram therapy.
PMID:29325448 Jukic MM et al; Am J Psychiatry. 2018 Jan 12:appiajp201717050550. doi: 10.1176/appi.ajp.2017.17050550. (Epub ahead of print)
Escitalopram is metabolized to S-demethylcitalopram (S-DCT) and S-didemethylcitalopram (S-DDCT). In humans, unchanged escitalopram is the predominant compound in plasma. At steady state, the concentration of the escitalopram metabolite S-DCT in plasma is approximately one-third that of escitalopram. The level of S-DDCT was not detectable in most subjects. In vitro studies show that escitalopram is at least 7 and 27 times more potent than S-DCT and S-DDCT, respectively, in the inhibition of serotonin reuptake, suggesting that the metabolites of escitalopram do not contribute significantly to the antidepressant actions of escitalopram. S-DCT and S-DDCT also have no or very low affinity for serotonergic (5-HT1-7) or other receptors including alpha- and beta-adrenergic, dopamine (D1-5), histamine (H1-3), muscarinic (M1-5), and benzodiazepine receptors. S-DCT and S-DDCT also do not bind to various ion channels including Na+, K+, Cl-, and Ca++ channels. In vitro studies using human liver microsomes indicated that CYP3A4 and CYP2C19 are the primary isozymes involved in the Ndemethylation of escitalopram.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
The elimination half-life of escitalopram is 27-32 hours, though this is increased by approximately 50% in the elderly and doubled in patients with reduced hepatic function. The elimination half-life of escitalopram's primary metabolite, S-desmethylcitalopram, is approximately 54 hours at steady state.
Biotransformation of escitalopram is mainly hepatic, with a mean terminal half-life of about 27-32 hours.
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
Escitalopram, like other selective serotonin re-uptake inhibitors, enhances serotonergic activity by binding to the orthosteric (i.e. primary) binding site on the serotonin transporter (SERT), the same site to which endogenous 5-HT binds, and thus prevents the re-uptake of serotonin into the presynaptic neuron. Escitalopram, along with [paroxetine], is also considered an allosteric serotonin re-uptake inhibitor - it binds to a secondary allosteric site on the SERT molecule to more strongly inhibit 5-HT re-uptake. Its combination of orthosteric and allosteric activity on SERT allows for greater extracellular 5-HT levels, a faster onset of action, and greater efficacy as compared to other SSRIs. The sustained elevation of synaptic 5-HT eventually causes desensitization of 5-HT1A auto-receptors, which normally shut down endogenous 5-HT release in the presence of excess 5-HT - this desensitization may be necessary for the full clinical effect of SSRIs and may be responsible for their typically prolonged onset of action. Escitalopram has shown little-to-no binding affinity at a number of other receptors, such as histamine and muscarinic receptors, and minor activity at these off-targets may explain some of its adverse effects.
The mechanism of antidepressant action of escitalopram, the S-enantiomer of racemic citalopram, is presumed to be linked to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT).
NIH; DailyMed. Current Medication Information for Lexapro (Escitalopram Oxalate) Tablet, Film Coated; Lexapro (Escitalopram Oxalate) Solution (Updated: January 26, 2017). Available from, as of March 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a
























