




1. 1206102-11-5
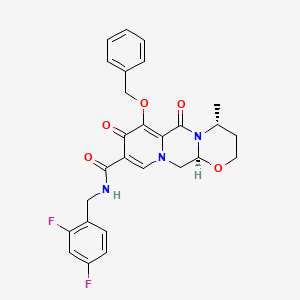
2. (4r,12as)-7-(benzyloxy)-n-(2,4-difluorobenzyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxamide
3. Dolutegravir O-benzyl Impurity
4. Sj4gz8739l
5. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-7-methyl-9,12-dioxo-11-phenylmethoxy-4-oxa-1,8-diazatricyclo[8.4.0.03,8]tetradeca-10,13-diene-13-carboxamide
6. (4r,12as)-7-(benzyloxy)-n-(2,4-difluorobenzyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
7. (4r,12as)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-4-methyl-6,8-dioxo-7-(phenylmethoxy)-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
8. 2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide, N-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-4-methyl-6,8-dioxo-7-(phenylmethoxy)-, (4r,12as)-
9. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-4-methyl-6,8-dioxo-7-(phenylmethoxy)-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
10. (4r,12as)-n-(2,4-difluorobenzyl)-7-benzylhydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
11. Unii-sj4gz8739l
12. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-4-methyl-6,8-dioxo-7-(phenylmethox
13. Schembl2302444
14. Mfcd22741602
15. Akos027323537
16. Zinc118591738
17. Ac-28372
18. Ds-11349
19. Cs-0158235
20. F11519
21. A852332
22. (4r,12as)-7-(benzyloxy)-n-(2,4-difluorobenzyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-
23. (4r,12as)-n-(2,4-difluorobenzyl)-7-benzylhydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1',2'
24. (4r,9as)-5-benzyloxy-n-(2,4-difluorobenzyl)-3,4,6,9,9a,10-hexahydro-4-methyl-6,10-dioxo-2h-1-oxa-4a,8a-diazaanthracene-7-carboxamide
| Molecular Weight | 509.5 g/mol |
|---|---|
| Molecular Formula | C27H25F2N3O5 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 88.2 |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 979 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |








