




1. 110931-77-6
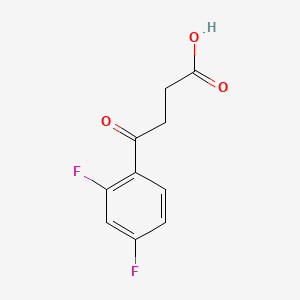
2. 4-(2,4-difluorophenyl)-4-oxobutyric Acid
3. 3-(2,4-difluorobenzoyl)propionic Acid
4. 3-(2',4'-difluorobenzoyl)propionic Acid
5. Mfcd00143016
6. 4-(2,4-difluorophenyl)-4-oxobutanoicacid
7. Schembl3527017
8. Posaconazole Related Compound 1
9. Dtxsid00378877
10. Zinc2512396
11. Ab3648
12. Akos000156915
13. As-65009
14. Db-028590
15. Cs-0130721
16. D4585
17. Ft-0657787
18. 4-(2,4-difluorophenyl);-4-oxobutanoic Acid
19. En300-50232
20. 3-(perfluorooctyl)-2-hydroxypropylacrylate
21. 931d776
22. A802269
23. Z607822476
24. 4-[2,4-bis(fluoranyl)phenyl]-4-oxidanylidene-butanoic Acid
| Molecular Weight | 214.16 g/mol |
|---|---|
| Molecular Formula | C10H8F2O3 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 214.04415044 g/mol |
| Monoisotopic Mass | 214.04415044 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 255 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




