



1. 109232-37-3
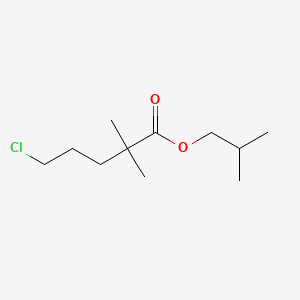
2. 2-methylpropyl 5-chloro-2,2-dimethylpentanoate
3. Isobutyl 5-chloro-2,2-dimethylpentanoate
4. 5-chloro-2,2-dimethyl-pentanoic Acid Isobutyl Ester
5. Isobutyl5-chloro-2,2-dimethylvalerate
6. Isobutyl 5-chloro-2,2-dimethyl Valerate
7. Pentanoic Acid, 5-chloro-2,2-dimethyl-, 2-methylpropyl Ester
8. Gemfibrozil Impurity 2
9. Ec 600-906-1
10. 5-chloro-2,2-dimethyl-pentanoicacidisobutylester
11. Schembl357604
12. Dtxsid60552414
13. Zinc2507097
14. Mfcd03265308
15. Akos015900638
16. Ab13858
17. Isobutyl-5-chloro-2,2-dimethylvalerate
18. Ds-13822
19. Isobutyl 5-chloro-2,2-di-methylpentanoate
20. Db-005440
21. Cs-0130724
22. Ft-0602434
23. 232i373
| Molecular Weight | 220.73 g/mol |
|---|---|
| Molecular Formula | C11H21ClO2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 7 |
| Exact Mass | 220.1230076 g/mol |
| Monoisotopic Mass | 220.1230076 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 176 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




