




1. 90357-51-0
2. N-[4-cyano-3-(trifluoromethyl)phenyl]methacrylamide Epoxide
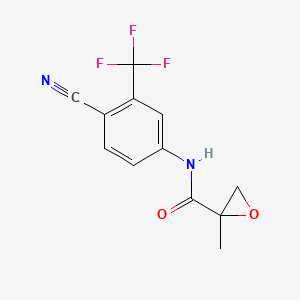
3. N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamide
4. N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-2-oxiranecarboxamide
5. Oxiranecarboxamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-
6. Bicalutamide Epoxide Impurity
7. 4-cyano-n-(2,3-epoxy-2-methylpropionyl)-3-trifluoromethylaniline
8. Mfcd08460201
9. N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-2-oxirane Carboxamide
10. N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-oxirane-2-carboxamide
11. 1,2-epoxy-2-methyl-n-[4-cyano-3-(trifluoromethyl)-phenyl]propionamide
12. Schembl202481
13. Dtxsid60436169
14. Akos015900542
15. Ac-1069
16. Ds-3154
17. Sy033473
18. Am20041370
19. C3607
20. Cs-0153887
21. 357c510
22. Q-100884
23. N-[4-cyano-3-trifluoromethylphenyl]-2-methyloxiranecaboxamide
24. Cyano-3-(trifluoromethyl)phenyl]-2-methyl-2-oxirane Carboxamide
25. N-[3-(trifluoromethyl)-4-cyanophenyl]-2-methyloxirane-2-carboxamide
26. 2-methyl-oxirane-2-carboxylic Acid (4-cyano-3-trifluoromethyl-phenyl)-amide
27. N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-2-oxiranecarbox-amide
28. N-(4-cyano-3-(trifluoromethyl)phenyl)methacrylamide;n-(4-cyano-3-(trifluoromethyl)phenyl)-2-methyloxirane-2-carboxamide
| Molecular Weight | 270.21 g/mol |
|---|---|
| Molecular Formula | C12H9F3N2O2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 270.06161202 g/mol |
| Monoisotopic Mass | 270.06161202 g/mol |
| Topological Polar Surface Area | 65.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






