



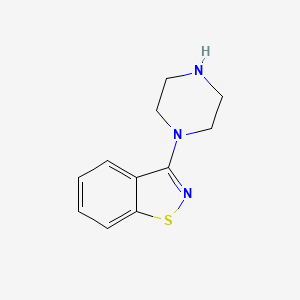

1. 87691-87-0
2. 3-(piperazin-1-yl)benzo[d]isothiazole
3. 3-piperazin-1-yl-benzo[d]isothiazole
4. 3-piperazin-1-yl-1,2-benzothiazole
5. Bitp
6. 1,2-benzisothiazole, 3-(1-piperazinyl)-
7. N-(3-benzisothiazolyl)piperazine
8. Mfcd04117970
9. 3-(piperazin-1-yl)-1,2-benzothiazole
10. 3-(1-piperazinyl)benzisothiazole
11. 3-piperazin-1-yl-1,2-benzisothiazole
12. 3-piperazin-1-yl-benzo(d)isothiazole
13. 3-(piperazin-1-yl)benzo(d)isothiazole
14. Chembl1154
15. Ziprasidone Related Compound A Free Base
16. Id-11614
17. Mj-14069
18. 59d8rat1f9
19. 3-piperazino-1,2-benzisothiazole
20. Id11614
21. 3-piperazin-1-ylbenzo[d]isothiazole
22. Unii-59d8rat1f9
23. 3-piperazinylbenzo[d]isothiazole
24. 3-piperazinyl-1,2-benzisothiazole
25. 4-(1,2-benzisothiazol-3-yl)-1-piperazine
26. Benzisothiazylpiperazine
27. Ziprasidone (m5)
28. Piperazinyl Benzoisothiazole
29. N-benzisothiazolylpiperazine
30. 3-piperazinyl-benzisothiazole
31. Schembl250876
32. Lurasidone Metabolite M1
33. N-(3benzoisothiazolyl)piperazine
34. 3-piperazin-1-yl-benzisothiazole
35. Dtxsid70236556
36. 3-piperazinyl-1,2-benzisothiazol
37. Chebi:191681
38. N-(3-benzoisothiazolyl)piperazine
39. N-(3-benzisothiazolyl) Piperazine
40. N-(3-benzisothiazolyl)-piperazine
41. 3-piperazinylbenzo [d] Isothiazole
42. Albb-008924
43. Bcp17556
44. Zinc2568290
45. Ziprasidone Hydrochloride Monohydrate Specified Impurity A [ep]
46. Bdbm50007695
47. Stk347701
48. Akos000301991
49. Ab20773
50. Ac-1868
51. Ccg-275122
52. Cs-w010470
53. Ds-1936
54. Gf-0131
55. 3-(1-piperazinyl)-i,2-benzisothiazole
56. 4-(1,2-benzisothiazol-3-yl)piperazine
57. N-(1,2-benzisothiazol-3-yl)piperazine
58. 1-(1,2-benzoisothiazol-3-yl)piperazine
59. 4-(1,2-benzisothiazole-3-yl)piperazine
60. 1-(1,2-benzisothiazol-3-yl) Piperazine
61. 4-(1,2-benzisothiazol-3-yl)-piperazine
62. Sy047003
63. 1-(1,2-benzoisothiazol- 3-yl)piperazine
64. Db-006229
65. Am20060794
66. Bb 0216394
67. Ft-0601156
68. Ft-0650663
69. P2064
70. 1-(1,2-benzisothiazol-3-yl)piperazine
71. Benzisothiazole-3-yl-piperazine (bitp)
72. 691p870
73. A1-00278
74. W-205602
75. Q27261694
76. F0001-2180
77. 3-(1-piperazinyl)-1,2-benzisothiazole, >=98% (hplc)
78. Ziprasidone Mesilate Trihydrate Impurity A [ep Impurity]
79. 3-(piperazin-1-yl)benzo[d]isothiazole;3-piperazinyl-1,2-benzisothiazole
80. Ziprasidone Hydrochloride Monohydrate Impurity A [ep Impurity]
| Molecular Weight | 219.31 g/mol |
|---|---|
| Molecular Formula | C11H13N3S |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 219.08301860 g/mol |
| Monoisotopic Mass | 219.08301860 g/mol |
| Topological Polar Surface Area | 56.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 218 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
3-(1-Piperazinyl)-1,2-benzisothiazole is a known human metabolite of Perospirone and ziprasidone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560










