

X



1. 864070-18-8
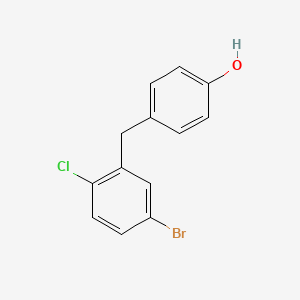
2. 4-[(5-bromo-2-chlorophenyl)methyl]phenol
3. Phenol, 4-[(5-bromo-2-chlorophenyl)methyl]-
4. Dapagliflozin Impurity 39
5. Schembl17842
6. Dtxsid40726338
7. Amy31452
8. Mfcd19441145
9. Zinc85224782
10. 4-(5-bromo-2-chloro-benzyl)-phenol
11. Akos016008069
12. Sb17546
13. Ac-29064
14. As-10217
15. Da-22435
16. 4-[(5-bromo-2-chloro-phenyl)methyl]phenol
17. Cs-0141423
18. Ft-0718315
19. C76807
| Molecular Weight | 297.57 g/mol |
|---|---|
| Molecular Formula | C13H10BrClO |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 295.96036 g/mol |
| Monoisotopic Mass | 295.96036 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



