




1. 80036-89-1
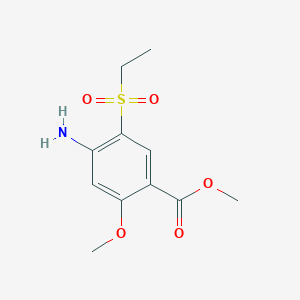
2. Methyl 4-amino-5-ethylsulfonyl-2-methoxybenzoate
3. Methyl 4-amino-5-(ethylsulphonyl)-2-methoxybenzoate
4. Methyl 4-amino-5-(ethanesulfonyl)-2-methoxybenzoate
5. Mfcd07787259
6. 2-methoxy-4-amino-5-ethsulfonyl Benzoic Acid Methyl Ester
7. Schembl12070949
8. Dtxsid00512653
9. Bcp22950
10. Ac2435
11. Zinc16123605
12. Akos015843676
13. Ac-6118
14. Ps-7264
15. Sy042588
16. Ft-0645038
17. A839813
18. Methyl4-amino-5-(ethylsulfonyl)-2-methoxybenzoate
19. 2-methoxyl-4-amino-5-ethylsulfonyl Methyl Benzoate
20. J-522301
| Molecular Weight | 273.31 g/mol |
|---|---|
| Molecular Formula | C11H15NO5S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 273.06709375 g/mol |
| Monoisotopic Mass | 273.06709375 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 389 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




