




1. 766-36-9
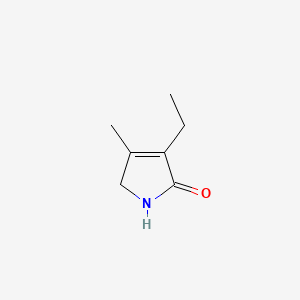
2. 3-ethyl-4-methyl-3-pyrroline-2-one
3. 3-ethyl-4-methyl-2,5-dihydro-1h-pyrrol-2-one
4. 4-ethyl-3-methyl-1,2-dihydropyrrol-5-one
5. 2h-pyrrol-2-one, 3-ethyl-1,5-dihydro-4-methyl-
6. 3-ethyl-4-methyl-1h-pyrrol-2(5h)-one
7. 1,5-dihydro-3-ethyl-4-methyl-2h-pyrrol-2-one
8. Mfcd00173861
9. 3-ethyl-4-methyl-2-oxopyrroline
10. Ec 616-364-4
11. Schembl1430224
12. Yctntsvmjwiytq-uhfffaoysa-
13. 3-ethyl-4-methylpyrroline-2-one
14. Dtxsid70357558
15. Zinc404205
16. Bcp28365
17. Akos006223717
18. Ab04375
19. Am62801
20. Cs-w002472
21. Ac-23984
22. As-12826
23. Sy032675
24. Db-006425
25. 3-ethyl-4-methyl-3-pyrrolin-2-one, 97%
26. A9711
27. E0964
28. Ft-0600899
29. Ft-0667991
30. 766e369
31. 3-ethyl-4-methyl-2,5-dihydro-1h-pyrroline-2-one
32. J-512425
33. 3-ethyl-1,5-dihydro-4-methyl-2h-pyrrol-2-one;
| Molecular Weight | 125.17 g/mol |
|---|---|
| Molecular Formula | C7H11NO |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 125.084063974 g/mol |
| Monoisotopic Mass | 125.084063974 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





