


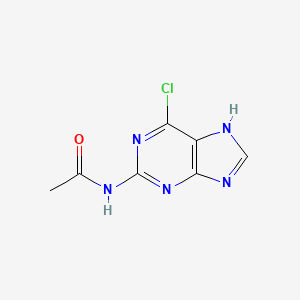

1. 2-acetamido-6-chloropurine
2. 7602-01-9
3. N-(6-chloro-9h-purin-2-yl)acetamide
4. Nciopen2_003279
5. Schembl5197095
6. Dtxsid60997366
7. 2-acetylamino-6-chloro-9h-purine
8. Bcp18787
9. Nsc69879
10. Zinc6761421
11. Mfcd00823609
12. Nsc-69879
13. 2-(acetylamino)-6-chloro-9h-purine
14. Akos015912768
15. Akos037621661
16. 2-(acetylamino)-6-chloropurine
17. Bs-29485
18. Cs-0067220
19. Ft-0713232
20. N-(6-chloro-3h-purin-2-yl)ethanimidic Acid
21. C76431
22. A915106
23. W-200610
24. 5-pyrimidinecarbonitrile, 1,6-dihydro-2-(methylthio)-6-oxo-
| Molecular Weight | 211.61 g/mol |
|---|---|
| Molecular Formula | C7H6ClN5O |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 211.0260875 g/mol |
| Monoisotopic Mass | 211.0260875 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 238 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




