



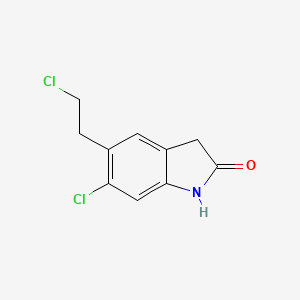

1. 118289-55-7
2. 5-chloroethyl-6-chloro-1,3-dihydro-2h-indole-2-one
3. 6-chloro-5-(2-chloroethyl)-1,3-dihydro-2h-indol-2-one
4. 6-chloro-5-(2-chloroethyl)oxindole
5. 6-chloro-5-(2-chloroethyl)-1,3-dihydroindol-2-one
6. 5-chloroethyl-6-chloro-1,3-dihydro-1h-indol-2-one
7. 2h-indol-2-one, 6-chloro-5-(2-chloroethyl)-1,3-dihydro-
8. 5-(2-chloroethyl)-6-chlorooxindole
9. Zt4r5s724x
10. Ziprasidone Impurity F (5-(2-chloroethyl)-6-chlorooxindole)
11. 6-chloro-5-(2-chloroethyl)-2-indolinone
12. Ec 421-320-0
13. Unii-zt4r5s724x
14. Schembl623364
15. Dtxsid50442471
16. Bcp11915
17. Zinc2524898
18. Bbl029977
19. Mfcd03411598
20. Stl373352
21. Zb1472
22. 6-chloro-5-(2-chloroethyl)-1,3
23. 5-(2-chloroethyl)-6-chloro Oxindole
24. 5-(2-chloroethyl)-6-chloro-oxindole
25. 6-chloro-5-(2-chloroethyl) Oxindole
26. Akos015889238
27. Ab14169
28. Ac-5337
29. Cs-w010382
30. 5-(2-chloro Ethyl)-6-chloro Oxindole
31. 5-(2-chloroethyl) -6-chloro Oxindole
32. As-11846
33. 5-(2-chloroethyl)-6-chloroindoline-2-one
34. 5-chloroethyl-6-chloroindole-2-one
35. Bb 0258985
36. Ft-0642887
37. 5-(2-chloro Ethyl)-6-chlorooxindole
38. 6-chloro-5-(2-chloroethyl)-2h-indol-2-one
39. A22730
40. F14939
41. 289c557
42. 5-chloroethyl-6-chloro-1,3-dihydro-indol-2-one
43. 6-chloro-5-(2-chloroethyl)-2-oxoindoline
44. J-509563
45. 6-chloro-5-(2-chloro-ethyl)- 1,3-dihydro-indol-2-one
46. 6-chloro-5-(2-chloro-ethyl)-1,3-dihydro-indol-2-one
47. (6-chloro-5-(2-chloroethyl)-1,3-dihydro-2h-indol-2-one)
48. 2h-indol-2-one,6-chloro-5-(2-chloroethyl)-1,3-dihydro-
49. 5-(2-chloroethyl)-6-chloro-1,3-dihydro-2h-indol-2-one
50. 6-chloro-5-(2-chloroethyl)-1,3-dihydro-2h-indole-2-one
| Molecular Weight | 230.09 g/mol |
|---|---|
| Molecular Formula | C10H9Cl2NO |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 229.0061193 g/mol |
| Monoisotopic Mass | 229.0061193 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 234 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







