



1. 54197-66-9
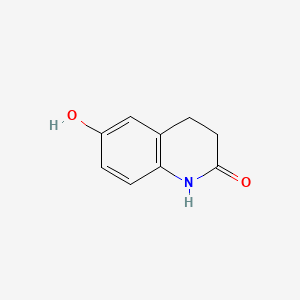
2. 6-hydroxy-3,4-dihydroquinolin-2(1h)-one
3. 6-hydroxy-3,4-dihydro-1h-quinolin-2-one
4. 6-hydroxy-2-oxo-1,2,3,4-tetrahydroquinoline
5. 6-hydroxy-2(1h)-3,4-dihydroquinolinone
6. 3,4-dihydro-6-hydroxy-2(1h)-quinolinone
7. 6-hydroxy-3,4-dihydrocarbostyril
8. 2(1h)-quinolinone, 3,4-dihydro-6-hydroxy-
9. Cilostazol Related Compound A
10. 6-hydroxy-3,4-dihydro-1h-quinoline-2-one
11. 3,4-dihydro-6-hydroxycarbostyril
12. 6-hydroxy-3,4-dihydroquinolinone
13. 6-hydroxy-1,2,3,4-tetrahydro-2-quinolinone
14. 2c5ndt39oc
15. 2-oxo-1,2,3,4-tetrahydroquinolin-6-ol
16. Chembl2430710
17. Mfcd02179410
18. 6-hydroxy-3,4-dihydro-1h Quinolin-2-one
19. 6-hydroxyl-3.4-dihydrocarbostyril
20. Unii-2c5ndt39oc
21. 6-hydroxyl
22. Cilostazol Related Compound A [usp]
23. 6-hydroxy-1,3,4-trihydroquinolin-2-one
24. Ec 611-111-4
25. Schembl426336
26. 3,4-dihydro-2,6-quinolinediol
27. Dtxsid60202548
28. 6-hydroxyl-3,4-dihydrocarbostyril
29. 6-hydroxy-3,4-dihydro-carbostyril
30. Bcp22789
31. Zinc3957998
32. 3,4-dihydro-6-hydroxy-carbostyril;
33. 6-hydroxy-3, 4-dihydro-carbostyril
34. Ac-498
35. Bdbm50493468
36. 6-hydroxy-3,4-dihydro-2-quinolinone
37. Akos008090878
38. Bcp9000194
39. Cs-w002905
40. Fs-1287
41. 6-hydroxyl-3,4-dihydroquinoline-2-one
42. 6-hydroxy-3,4dihydroquinolin-2(1h)-one
43. Bp-10200
44. Sy002022
45. 34-dihydro-6-hydroxy-2(1h)-quinolinone
46. 3,4-dihydro-6-hydroxyquinolin-2(1h)-one
47. 6-hydroxy-3,4-dihydro-2-(1h)-quinolone
48. Db-002613
49. 3,4-dihydro-6-hydroxy-2-(1h)-quinolinone
50. 6-hydroxy-3,4-dihydro-2(1 H)-quinolinone
51. Am20061119
52. D3448
53. Ft-0640226
54. 6-hydroxy-1,2,3,4-tetrahydroquinolin-2-one
55. En300-27032
56. 6-hydroxy-3,4-dihydro-(1h)-quinoline-2-one
57. Cilostazol Related Compound A [usp-rs]
58. 6-hydroxy-2-oxo-1,2,3,4-tetrahydro Quinoline
59. 197h669
60. 6-hydroxy-3,4-dihydro-2(1h)-quinolinone, 97%
61. 3,4-dihydro-6-hydroxycarbostyril-2(1h)quinolinone
62. Cilostazol Related Compound A [usp Impurity]
63. J-518785
64. Q27254550
65. Cilostazol Related Compound A, United States Pharmacopeia (usp) Reference Standard
66. 6-hydroxy-3,4-dihydroquinolin-2(1h)-one;3,4-dihydro-6-hydroxycarbostyril-2(1h)quinolinone; 6-hydroxy-3,4-dihydro-2(1h)-quinolinone
| Molecular Weight | 163.17 g/mol |
|---|---|
| Molecular Formula | C9H9NO2 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 163.063328530 g/mol |
| Monoisotopic Mass | 163.063328530 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





