




1. 536760-29-9
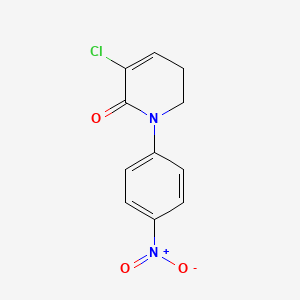
2. 3-chloro-5,6-dihydro-1-(4-nitrophenyl)-2(1h)-pyridinone
3. 5-chloro-1-(4-nitrophenyl)-2,3-dihydropyridin-6-one
4. 2(1h)-pyridinone, 3-chloro-5,6-dihydro-1-(4-nitrophenyl)-
5. 2(1h)-pyridine,3-chloro-5,6-dihydro-1-(4-nitrophenyl)
6. Apixaban Related Compound 2
7. Schembl8243895
8. Dtxsid90470632
9. Amy36801
10. Bcp11629
11. Cs-m0373
12. Mfcd18207128
13. Zinc38247346
14. Akos015951085
15. Fd10599
16. Ac-26841
17. Da-16944
18. Ds-16656
19. Ft-0758197
20. A850072
21. J-512260
22. 3-chloro-5, 6-dihydro-1-(4-nitrophenyl)-2(1h)-pyridinone
| Molecular Weight | 252.65 g/mol |
|---|---|
| Molecular Formula | C11H9ClN2O3 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 252.0301698 g/mol |
| Monoisotopic Mass | 252.0301698 g/mol |
| Topological Polar Surface Area | 66.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 356 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







