
1. 2 Ketoglutarate
2. 2 Ketoglutaric Acid
3. 2 Oxoglutarate
4. 2 Oxoglutaric Acid
5. 2-ketoglutarate
6. 2-ketoglutaric Acid
7. 2-oxoglutarate
8. Alpha Ketoglutarate
9. Alpha Ketoglutaric Acid
10. Alpha Ketoglutaric Acid, Diammonium Salt
11. Alpha Ketoglutaric Acid, Dipotassium Salt
12. Alpha Ketoglutaric Acid, Disodium Salt
13. Alpha Ketoglutaric Acid, Monopotassium Salt
14. Alpha Ketoglutaric Acid, Monosodium Salt
15. Alpha Ketoglutaric Acid, Potassium Salt
16. Alpha Ketoglutaric Acid, Sodium Salt
17. Alpha Oxoglutarate
18. Alpha-ketoglutarate
19. Alpha-ketoglutarate, Calcium
20. Alpha-ketoglutaric Acid
21. Alpha-ketoglutaric Acid, Calcium Salt (2:1)
22. Alpha-ketoglutaric Acid, Diammonium Salt
23. Alpha-ketoglutaric Acid, Dipotassium Salt
24. Alpha-ketoglutaric Acid, Disodium Salt
25. Alpha-ketoglutaric Acid, Monopotassium Salt
26. Alpha-ketoglutaric Acid, Monosodium Salt
27. Alpha-ketoglutaric Acid, Potassium Salt
28. Alpha-ketoglutaric Acid, Sodium Salt
29. Alpha-oxoglutarate
30. Calcium Alpha Ketoglutarate
31. Calcium Alpha-ketoglutarate
32. Calcium Ketoglutarate
33. Ketoglutaric Acid
34. Ketoglutaric Acids
35. Oxogluric Acid
36. Oxoglutarates
1. 2-ketoglutaric Acid
2. 2-oxopentanedioic Acid
3. 328-50-7
4. Alpha-ketoglutaric Acid
5. Alpha-ketoglutarate
6. 2-oxoglutarate
7. Oxoglutaric Acid
8. Alpha Ketoglutarate
9. Oxoglutarate
10. 2-oxo-1,5-pentanedioic Acid
11. Pentanedioic Acid, 2-oxo-
12. Alphaketoglutaric Acid
13. Alpha-oxoglutaric Acid
14. Oxoglurate
15. Glutaric Acid, 2-oxo-
16. Oxogluric Acid
17. Glutaric Acid, Alpha Keto
18. 2-ketoglutarate
19. Pentanedioic Acid, Oxo-
20. A-ketoglutaric Acid
21. Ketoglutaric Acid
22. Bis(l-arginin)-2-oxoglutarat
23. Oxoglurate [inn]
24. Alpha Ketoglutaric Acid
25. .alpha.-ketoglutaric Acid
26. Ai3-26938
27. Mfcd00004165
28. Nsc 17391
29. Alpha-ketoglutaric Acid Alpha
30. 17091-15-5
31. 2-oxo-pentanedioic Acid
32. Alpha-keto-glutaric Acid
33. Nsc17391
34. .alpha.-oxoglutaric Acid
35. Nsc-17391
36. 378-50-7
37. Chembl1686
38. Chebi:30915
39. Glutaric Acid, 2-oxo- (8ci)
40. 8id597z82x
41. 2-oxopentanedioate
42. 2-oxo-glutaric Acid
43. 2-oxopentanedionate
44. 2-ketoglutaricacid
45. Dsstox_cid_13179
46. Dsstox_rid_79054
47. Dsstox_gsid_33179
48. Pentanedioic Acid, 2-oxo-, Sodium Salt
49. Ketoglutaric Acids
50. Cas-328-50-7
51. Akg
52. Einecs 206-330-3
53. Alpha-ketoglutaricum Acidum
54. Unii-8id597z82x
55. 3ouj
56. 4nro
57. 4oct
58. 4qkd
59. 4usi
60. Keto Glutaric Acid
61. 2-oxoglutaric-acid
62. .alpha.-ketoglutarate
63. 2-oxo-pentanedioicaci
64. 34410-46-3
65. Alpha-oxo-glutaric Acid
66. Alpha -ketoglutaric Acid
67. Alpha-oxopentanedioic Acid
68. 2-oxo-1,5-pentanedioate
69. Bmse000064
70. Bmse000801
71. Bmse000937
72. Schembl7400
73. Ncistruc1_001710
74. Ncistruc2_000215
75. Alfa-ketoglutaric Acid
76. A-ketoglutaricum Acidum
77. Glutaric Acid, .alpha.-keto-
78. Gtpl3636
79. Oxogluric Acid [mart.]
80. Dtxsid5033179
81. Ketoglutaric Acid [inci]
82. Oxogluric Acid [who-dd]
83. Nci17391
84. Pentanedioic Acid, 2-oxo- (9ci)
85. Zinc1532519
86. Tox21_110016
87. Tox21_200918
88. Bbl010614
89. Bdbm50303766
90. Ccg-37641
91. Ncgc00013225
92. S6237
93. Stk002174
94. 2-oxopentanedioic Acid [fhfi]
95. A-ketoglutaricum Acidum [hpus]
96. Akos000120908
97. Cs-w014352
98. Db02926
99. Db03806
100. Db08845
101. Hy-w013636
102. .alpha.-ketoglutaric Acid [mi]
103. Ncgc00013225-01
104. Ncgc00013225-02
105. Ncgc00013225-03
106. Ncgc00013225-04
107. Ncgc00013225-05
108. Ncgc00013225-06
109. Ncgc00090946-01
110. Ncgc00090946-02
111. Ncgc00258472-01
112. Alpha-ketoglutaric Acid, >=99.0% (t)
113. As-12579
114. Nci60_001411
115. Sy004310
116. Db-048280
117. Am20090486
118. Ft-0612734
119. Ft-0670633
120. K0005
121. C00026
122. D70548
123. K-2400
124. Ab00640269-03
125. A855282
126. Q306140
127. Alpha-ketoglutaric Acid, >=98.5% (naoh, Titration)
128. Z57127547
129. F2191-0182
130. 15118487-024f-412c-9995-24e8e6ca72ef
131. Alpha-ketoglutaric Acid, Bioreagent, Suitable For Cell Culture, Suitable For Insect Cell Culture
| Molecular Weight | 146.10 g/mol |
|---|---|
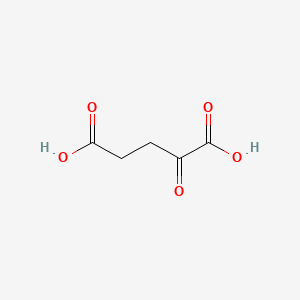
| Molecular Formula | C5H6O5 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 146.02152329 g/mol |
| Monoisotopic Mass | 146.02152329 g/mol |
| Topological Polar Surface Area | 91.7 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 171 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
--Ketoglutarate is not approved for any indication in the world but is an investigational drug in the United States. The potential indications for -Ketoglutarate are in patients with the metabolic disorder propionic acidemia and in trauma patients with muscle loss.
All of the physiological roles of alpha-ketoglutarate have not been determined. What is known is that alpha-keotglutarate is involved in the Krebs cycle, transamination reactions, and promotes muscle synthesis.
The exact mechanisms of action for -Ketoglutarate have not yet been elucidated. Some of -Ketoglutarates actions include acting in the Krebs cycle as an intermediate, transamination reactions during the metabolism of amino acids, forming glutamic acid by combining with ammonia, and reducing nitrogen by combining with it as well. Concerning -Ketoglutarates actions with ammonia, it is proposed that -Ketoglutarate can help patients with propionic academia who have high levels of ammonia and low levels of glutamine/glutamate in their blood. Because endogenous glutamate/glutamine is produced from -Ketoglutarate, propionic acidemia patients have impaired production of -Ketoglutarate and supplementation of -Ketoglutarate should improve the condition of these patients. Several other experimental studies have also shown that administration of -Ketoglutarate in parenteral nutrition given to post-operative patients helped attenuate the decreased synthesis of muscle protein that is often seen after a surgery. This decreased muscle synthesis is speculated to be due to too low -Ketoglutarate levels.
MARKET PLACE

Reply
10 Jul 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
