
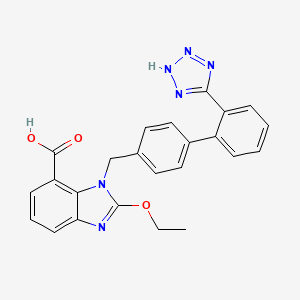

1. 2-ethoxy-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-1h-benzimidazole-7-carboxylic Acid
2. 2-ethoxy-7-carboxy-1-(2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methylbenzimidazole
3. Cv 11974
4. Cv-11974
5. Cv11974
1. 139481-59-7
2. Blopress
3. Cv-11974
4. 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylic Acid
5. Cv 11974
6. 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid
7. 2-ethoxy-1-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-7-benzimidazolecarboxylic Acid
8. Nsc-759858
9. Candesartan Cilexetil Related Compound G
10. Chembl1016
11. S8q36md2xx
12. Chebi:3347
13. Ncgc00167474-01
14. Dsstox_cid_2725
15. Dsstox_rid_75453
16. Dsstox_gsid_22725
17. 1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylic Acid
18. 1h-benzimidazole-7-carboxylic Acid, 2-ethoxy-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-
19. [3h]candesartan
20. 1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylic Acid
21. 2-(ethyloxy)-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-benzimidazole-7-carboxylic Acid
22. 2-ethoxy-1-({2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl}methyl)-1h-benzimidazole-7-carboxylic Acid
23. Candesartan [inn]
24. Cas-139481-59-7
25. Sr-05000001447
26. Candesartan (usan/inn)
27. Unii-s8q36md2xx
28. Candesartan [usan:inn:ban]
29. Hsdb 7520
30. 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid
31. 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]-4-benzimidazolecarboxylic Acid
32. Candesartan-[d5]
33. 2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-benzimidazole-7-carboxylic Acid
34. 2-ethoxy-3-[[4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid
35. Candesartan- Bio-x
36. Candemore
37. Celexetil
38. Candesartan (atacand)
39. Ks-5003
40. Candesartan - Atacand
41. Candesartan [mi]
42. Tcv-116 (prodrug)
43. Candesartan [hsdb]
44. Candesartan [usan]
45. Candesartan [vandf]
46. Ec 604-138-8
47. Schembl3938
48. 2-ethoxy-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-benzimidazole-7-carboxylic Acid
49. 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]-3h-benzoimidazole-4-carboxylic Acid
50. Candesartan [who-dd]
51. Gtpl587
52. Mls003915631
53. Mls004774041
54. Bidd:gt0350
55. Gtpl6907
56. Dtxsid0022725
57. Bcpp000302
58. Hms2089m22
59. Hms3651c13
60. Hms3715f13
61. Pharmakon1600-01502288
62. Act02607
63. Bcp01137
64. Hy-b0205
65. Str09609
66. Zinc3782818
67. Tox21_112478
68. Bdbm50240609
69. Mfcd00864463
70. Nsc755311
71. Nsc759858
72. S1578
73. Akos015888154
74. Akos025117340
75. Tox21_112478_1
76. Ab07470
77. Am84369
78. Bcp9000479
79. Ccg-213059
80. Ccg-269122
81. Db13919
82. Nsc 759858
83. Nsc-755311
84. Ncgc00167474-02
85. Ncgc00167474-04
86. Ncgc00167474-06
87. 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl] Methyl]-3h-benzoimidazole-4-carboxylic Acid
88. Bc164271
89. Smr002203608
90. Smr003500711
91. C-266
92. Ft-0602912
93. Sw199612-2
94. C07468
95. D00522
96. Ab01275447-01
97. Ab01275447_02
98. Ab01275447_03
99. 481c597
100. A807545
101. A808309
102. L000156
103. Q415970
104. Candesartan 100 Microg/ml In Acetonitrile:methanol
105. J-007281
106. Sr-05000001447-1
107. Sr-05000001447-2
108. Sr-05000001447-5
109. Candesartan Cilexetil Impurity G [ep Impurity]
110. Candesartan Cilexetil Related Compound G [usp-rs]
111. 2-ethoxy-1-[[2'-(1 H-tetrazol-5-yl)biphenyl-4-yl]methyl]-benzimidazole-7-carboxylic Acid
112. 2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]-benzimidazole-7-carboxylic Acid
113. 2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylic Acid
114. 2-ethoxy-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4ethyl]}-1h-benzimidazole-7-carboxylic Acid
115. 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid.
116. 2-ethoxy-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzimidazole-4-carboxylic Acid
117. 1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylicacid
118. 2-ethoxy-1-((2-(1h-tetrazole-5-yl)biphenyl-4-yl)methyl)-1h-benzamidazole-7-carboxylic Acid.
119. 2-ethoxy-1-({4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1h-1,3-benzodiazole-7-carboxylic Acid
120. 2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]-1h-benzimidazole-7-carboxylic Acid (candesartan)
121. 2-ethoxy-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-benzimidazole-7-carboxylic Acid
122. 2-ethoxy-1-{[2'-(2h-1,2,3,4-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}-1h-1,3-benzodiazole-7-carboxylic Acid
123. 2-ethoxy-3-[2''-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
124. 2-ethoxy-3-[2''-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid(cv-11974)
125. 2-ethoxy-3-[2''-(2h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
126. 2-ethoxy-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
| Molecular Weight | 440.5 g/mol |
|---|---|
| Molecular Formula | C24H20N6O3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 440.15968852 g/mol |
| Monoisotopic Mass | 440.15968852 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 660 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Candesartan. Online file (MeSH, 2014). Available from, as of September 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Atacand is indicated for the treatment of hypertension in adults and children 1 to < 17 years of age. It may be used alone or in combination with other antihypertensive agents. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Atacand is indicated for the treatment of heart failure (NYHA class II-IV) in adults with left ventricular systolic dysfunction (ejection fraction = 40%) to reduce cardiovascular death and to reduce heart failure hospitalizations. Atacand also has an added effect on these outcomes when used with an ACE inhibitor. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Both angiotensin II receptor antagonists and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2059
For more Therapeutic Uses (Complete) data for CANDESARTAN (7 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Atacand as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Atacand as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Neonates with a history of in utero exposure to Atacand: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
For more Drug Warnings (Complete) data for CANDESARTAN (19 total), please visit the HSDB record page.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA06 - Candesartan
The volume of distribution of candesartan is 0.13 L/kg. Candesartan is highly bound to plasma proteins (>99%) and does not penetrate red blood cells. The protein binding is constant at candesartan plasma concentrations well above the range achieved with recommended doses. In rats, it has been demonstrated that candesartan crosses the blood-brain barrier poorly, if at all. It has also been demonstrated in rats that candesartan passes across the placental barrier and is distributed in the fetus.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Following administration of candesartan cilexetil, the absolute bioavailability of candesartan was estimated to be 15%. After tablet ingestion, the peak serum concentration (Cmax) is reached after 3 to 4 hours. Food with a high fat content does not affect the bioavailability of candesartan after candesartan cilexetil administration.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Total plasma clearance of candesartan is 0.37 mL/min/kg, with a renal clearance of 0.19 mL/min/kg. When candesartan is administered orally, about 26% of the dose is excreted unchanged in urine. Following an oral dose of (14)C-labeled candesartan cilexetil, approximately 33% of radioactivity is recovered in urine and approximately 67% in feces. Following an intravenous dose of (14)C-labeled candesartan, approximately 59% of radioactivity is recovered in urine and approximately 36% in feces. Biliary excretion contributes to the elimination of candesartan.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
It is not known whether candesartan is excreted in human milk, but candesartan has been shown to be present in rat milk.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Candesartan cilexetil is rapidly and completely bioactivated by ester hydrolysis during absorption from the gastrointestinal tract to candesartan, a selective AT1 subtype angiotensin II receptor antagonist. Candesartan is mainly excreted unchanged in urine and feces (via bile). It undergoes minor hepatic metabolism by O-deethylation to an inactive metabolite.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Candesartan has known human metabolites that include (2S,3S,4S,5R)-6-[2-Ethoxy-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carbonyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid and 3-[[4-[2-[2-[(3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxyoxan-2-yl]tetrazol-5-yl]phenyl]phenyl]methyl]-2-ethoxy-1H-benzimidazol-3-ium-4-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life of candesartan is approximately 9 hours.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
Although these agents /angiotensin II receptor antagonists/ are similar to ACE inhibitors in that they decrease the effects of angiotensin II, rather than decreasing the formation of angiotensin II, drugs antagonize angiotensin II at the type I angiotensin receptor. This allows the drugs to inhibit the vasoconstrictive and aldosterone promoting effects of angiotensin II without interfering with bradykinin degradation, significantly reducing the adverse effects of cough and angioedema seen with ACE inhibitor therapy. ... /Angiotensin II receptor antagonists/
Goldfrank, L.R. (ed). Goldfrank's Toxicologic Emergencies. 7th Edition McGraw-Hill New York, New York 2002., p. 782
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Candesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathways for angiotensin II synthesis. There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Candesartan has much greater affinity (>10,000-fold) for the ATI receptor than for the AT2 receptor. Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because candesartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Candesartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation. Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of candesartan on blood pressure.
NIH; DailyMed. Current Medication Information for Atacand (Candesartan Cilexetil) Tablet (Revised: April 2013). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a73e1339-9643-4eea-2cbe-e879c88fb50e
BUILDING BLOCK

