



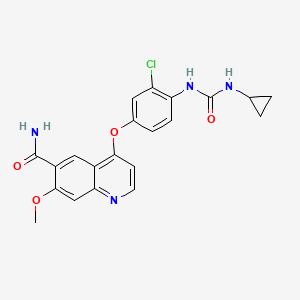

1. 4-(3-chloro-4-(((cyclopropylamino)carbonyl)amino)phenoxy)-7-hydroxy-6-quinolinecarboxamide
2. 4-(3-chloro-4-((cyclopropylaminocarbonyl)amino)phenoxy)-7-methoxy-6-quinolinecarboxamide
3. 4-(3-chloro-4-(n'-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide
4. E 7080
5. E-7080
6. E-7080 Mesylate
7. E7080
8. E7080 Mesylate
9. Er-203492-00
10. Lenvatinib Mesilate
11. Lenvatinib Mesylate
12. Lenvatinib Metabolite M2
13. Lenvatinib Methanesulfonate
14. Lenvima
15. N-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)oxy)-2-chlorophenyl)-n'-cyclopropylurea Monomethanesulfonate
1. 417716-92-8
2. E7080
3. 4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide
4. E7080 (lenvatinib)
5. Lenvatinib (e7080)
6. E-7080
7. E 7080
8. Er-203492-00
9. Lenvatinib Free Base
10. Unii-ee083865g2
11. 4-{3-chloro-4-[(cyclopropylcarbamoyl)amino]phenoxy}-7-methoxyquinoline-6-carboxamide
12. 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide
13. 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxyquinoline-6-carboxamide
14. Chembl1289601
15. Chebi:85994
16. 417716-92-8 (free Base)
17. Ee083865g2
18. 4-(3-chloro-4-(n'-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide
19. 4-(3-chloro-4-((cyclopropylaminocarbonyl)amino)phenoxy)-7-methoxy-6-quinolinecarboxamide
20. 4-[3-chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide
21. Lenvatinib [usan]
22. Lenvatinib [usan:inn]
23. Kisplyx
24. 4-(3-chloro-4-((cyclopropylcarbamoyl)amino)phenoxy)-7-methoxyquinoline-6-carboxamide
25. 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide
26. Lev
27. Lenvatinib; E7080
28. Lenvatinib [mi]
29. Lenvatinib Base- Bio-x
30. Lenvatinib [inn]
31. Lenvatinib (usan/inn)
32. Lenvatinib [who-dd]
33. Mls006011239
34. Schembl864638
35. Gtpl7426
36. Amy9240
37. Dtxsid50194605
38. Ex-a249
39. Bcpp000247
40. Hms3244a07
41. Hms3244a08
42. Hms3244b07
43. Hms3654a14
44. Bcp01799
45. Zinc3816292
46. Bdbm50331094
47. Mfcd16038644
48. Nsc755980
49. Nsc800781
50. S1164
51. Akos025401742
52. Bcp9000633
53. Ccg-264842
54. Cs-0109
55. Db09078
56. Nsc-755980
57. Nsc-800781
58. Sb16580
59. Ncgc00263198-01
60. Ncgc00263198-04
61. Ncgc00263198-07
62. Ac-25047
63. As-16203
64. Bl164616
65. Hy-10981
66. Smr004702999
67. Db-070219
68. Ft-0700727
69. Sw219259-1
70. D09919
71. 716c928
72. A825653
73. J-513372
74. Q6523413
75. Brd-k39974922-001-02-7
76. 4-[3-chloranyl-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
77. N-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)oxy)-2-chlorophenyl)-n'-cyclopropylurea
78. 4-[3-chloro-4-[[(cyclopropylamino)-oxomethyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide
79. 6-quinolinecarboxamide, 4-(3-chloro-4- (((cyclopropylamino)carbonyl)amino)phenoxy)-7-methoxy-
80. 6-quinolinecarboxamide, 4-(3-chloro-4-(((cyclopropylamino)carbonyl)amino)phenoxy)- 7-methoxy-
81. 6-quinolinecarboxamide, 4-(3-chloro-4-(((cyclopropylamino)carbonyl)amino)phenoxy)-7-methoxy-
| Molecular Weight | 426.9 g/mol |
|---|---|
| Molecular Formula | C21H19ClN4O4 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 426.1094828 g/mol |
| Monoisotopic Mass | 426.1094828 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 634 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lenvatinib is indicated for the treatment of following conditions. - Treatment of locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer. - Treatment of advanced renal cell carcinoma (RCC) in combination with everolimus following one prior antiangiogenic therapy. - First-line treatment of unresectable hepatocellular carcinoma (HCC).
FDA Label
Lenvima is indicated as monotherapy for the treatment of adult patients with progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hrthle cell) thyroid carcinoma (DTC), refractory to radioactive iodine (RAI).
Lenvima is indicated as monotherapy for the treatment of adult patients with advanced or unresectable hepatocellular carcinoma (HCC) who have received no prior systemic therapy.
Kisplyx is indicated for the treatment of adults with advanced renal cell carcinoma (RCC):
- in combination with pembrolizumab, as first-line treatment (see section 5. 1).
- in combination with everolimus, following one prior vascular endothelial growth factor (VEGF)-targeted therapy.
Treatment of follicular thyroid cancer , Treatment of osteosarcoma, Treatment of papillary thyroid cancer
Treatment of all conditions included in the category of malignant neoplasms except haematopoietic and lymphoid tissue neoplasms, papillary thyroid cancer , follicular thyroid cancer and osteosarcoma
Based on x-ray crystallography and kinetic interaction studies, lenvatinib binds to the adenosine 5'-triphosphate binding site of VEGFR2 and to a neighbouring region via a cyclopropane ring and thereby inhibits tyrosine kinase activity and associated signalling pathways.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE
L01XE29
L01XE29
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX08 - Lenvatinib
Absorption
Time to peak plasma concentration occurred from 1 to 4 hours postdose. Administration with food did not affect the extent of absorption, but decreased the rate of absorption and delayed the median Tmax from 2 hours to 4 hours.
Route of Elimination
Following administration of a radiolabeled dose, approximately 64% and 25% of the radiolabel were eliminated in the feces and urine, respectively.
Lenvatinib is metabolized by CYP3A and aldehyde oxidase.
The terminal elimination halflife of lenvatinib is approximately 28 hours.
Lenvatinib is a receptor tyrosine kinase (RTK) inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). Lenvatinib also inhibits other RTKs that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1, 2, 3, and 4; the platelet derived growth factor receptor alpha (PDGFR), KIT, and RET.



