



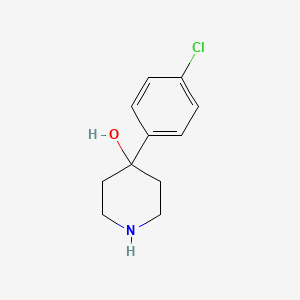

1. 4-(4'-chlorophenyl)-4-piperidinol
2. 4-cppo
3. Hcl Of 4-(4'-chlorophenyl)-4-piperidinol
1. 4-(4-chlorophenyl)piperidin-4-ol
2. 39512-49-7
3. 4-(4-chlorophenyl)-4-piperidinol
4. 4-piperidinol, 4-(4-chlorophenyl)-
5. 4-(p-chlorophenyl)piperidin-4-ol
6. 4-(p-chlorophenyl)-4-hydroxypiperidine
7. Mfcd00006001
8. 4-(chlorophenyl)-4-hydroxylpiperidine
9. 4-hydroxy-4-(4-chlorophenyl)piperidine
10. Und92fks0w
11. 4-(4'-chlorophenyl)-4-hydroxypiperidine
12. 4-hydroxy-4-(p-chlorophenyl)piperidine
13. 4-p-chlorophenyl-4-piperidinol
14. Chembl3298755
15. 4-(p-chlorophenyl)-4-piperidinol
16. Nsc-89568
17. 4-(4-chloro-phenyl)-piperidin-4-ol
18. 4-(para-chlorophenyl)-4-hydroxypiperidine
19. Haloperidol Metabolite I
20. Cphp
21. Unii-und92fks0w
22. Einecs 254-479-8
23. 4-(4'-chlorophenyl)-4-piperidinol
24. 4-(4-chlorophenyl)-4-hydroxy Piperidine
25. Maybridge1_002157
26. Dsstox_cid_31522
27. Dsstox_rid_97407
28. Dsstox_gsid_57733
29. Oprea1_639892
30. Schembl187716
31. 4-p-chlorophenylpiperidin-4-ol
32. Dtxsid7057733
33. Hms547k01
34. Lzayozufuamfld-uhfffaoysa-
35. 4-(4-chlorophenyl)piperidinol-4
36. Chebi:169225
37. 4-(4-chloropheny)-4-piperidinol
38. Zinc132448
39. Albb-006281
40. Bcp26969
41. Nsc89568
42. 4-(4- Chlorophenyl)-4-piperidinol
43. 4-(4-chlorophenyl)-piperidin-4-ol
44. Tox21_113829
45. 4-p-chlorophenyl-4-hydroxypiperidine
46. Bdbm50021772
47. Ccg-42197
48. Nsc 89568
49. Stk500351
50. 4-(chlorophenyl)-4-hydroxypiperidine
51. 4-(4-chlorophenyl)-4hydroxypiperidine
52. Akos000266656
53. 4-(4-chlorophenyl)-4-hydroxypiperdine
54. Ab00560
55. Ac-6533
56. Cs-w008038
57. Gf-0117
58. Sdccgmls-0065872.p001
59. 4-hydroxy-4-(p-chlorophenyl)-piperidine
60. Ncgc00253709-01
61. 4-(4- Chlorophenyl)-4-hydroxypiperidine
62. 4-(4-chlorophenyl) -4-hydroxypiperidine
63. Bp-12725
64. Sy048402
65. Cas-39512-49-7
66. A6608
67. Bb 0256874
68. C1291
69. Ft-0616612
70. O4-piperidinol, 4-(4-chlorophenyl)-
71. 4-(4-chlorophenyl)-4-hydroxypiperidine, 99%
72. 512c497
73. Sr-01000632212-1
74. W-106426
75. Brd-k34619475-001-01-6
76. Q27291160
77. Loperamide Hydrochloride Impurity C [ep Impurity]
| Molecular Weight | 211.69 g/mol |
|---|---|
| Molecular Formula | C11H14ClNO |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 211.0763918 g/mol |
| Monoisotopic Mass | 211.0763918 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 184 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
CPHP is a known human metabolite of reduced_haloperidol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs. 












