



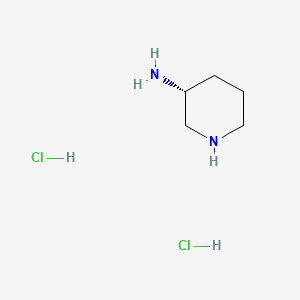

1. 334618-23-4
2. (r)-piperidin-3-amine Dihydrochloride
3. (r)-(-)-3-aminopiperidine Dihydrochloride
4. (r)-3-amino-piperidine Dihydrochloride
5. (r)-3-piperidinamine Dihydrochloride
6. (r)-3-aminopiperidine 2hcl
7. (r)-(-)-3-aminopiperidine 2hcl
8. (3r)-piperidin-3-amine;dihydrochloride
9. (r)-piperidine-3-amine Dihydrochloride
10. (3r)-piperidin-3-amine Dihydrochloride
11. 3-piperidinamine, Dihydrochloride, (3r)-
12. C5h14cl2n2
13. Mfcd06799458
14. R-3-aminopiperidine Dihydrochloride
15. R-3-aminopiperidine Dihcl
16. Schembl306090
17. Dtxsid40583408
18. 3-amino-piperidine-dihydrochloride
19. R-3-amino Piperidine Dihcl
20. Act04891
21. Cs-b0670
22. (r)-piperidin-3-aminedihydrochloride
23. 3-(r)-aminopiperidine Dihydrochloride
24. Akos015845481
25. Ac-6185
26. Ds-0861
27. Ps-8651
28. R18a234
29. (r)-3-amino-piperidine, Dihydrochloride
30. Db-024517
31. A5960
32. Am20090508
33. (3r)-3-aminopiperidine Dihydrochloride
34. (r)-piperidin-3-amine Dihydrochloriide
35. (r)-(-)-3-aminopiperidine Dihydrochloride, 97%
36. (r)-(-)-3-piperidinamine Dihydrochloride
37. (3r)-piperidin-3-amine--hydrogen Chloride (1/2)
38. J-650012
39. J-650226
40. W-202369
41. (r)-(-)-3-aminopiperidine Dihydrochloride, >=98.0% (gc)
| Molecular Weight | 173.08 g/mol |
|---|---|
| Molecular Formula | C5H14Cl2N2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 172.0534038 g/mol |
| Monoisotopic Mass | 172.0534038 g/mol |
| Topological Polar Surface Area | 38 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 54 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
















