




1. 24065-33-6
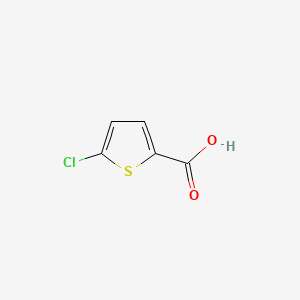
2. 5-chloro-2-thiophenecarboxylic Acid
3. 2-thiophenecarboxylic Acid, 5-chloro-
4. Mfcd00041426
5. Xv5src3pj3
6. 5-chloro Thiophene-2-carboxylic Acid
7. Chembl4280438
8. 5-chloro-2-thiophene Carboxylic Acid
9. Nsc-14776
10. Nsc 14776
11. Brn 0118361
12. Ai3-61740
13. 5-chlorothiophene-2-carboxylicacid
14. Nsc14776
15. Rivaroxaban Impurity C
16. Maybridge3_003717
17. Unii-xv5src3pj3
18. Rivaroxaban Related Compound
19. Schembl77196
20. 5-18-06-00177 (beilstein Handbook Reference)
21. 5-chlorothiophenecarboxylic Acid
22. Rivaroxaban Ctca Impurity
23. Dtxsid10178800
24. 5-chloro-2-thiophenecarboxylicacid
25. Hms1441i21
26. Zinc125451
27. 5-chloro-2-thiophencarboxylic Acid
28. 5-chlorthiophene-2-carboxylic Acid
29. 2-chloro-5-carboxythiophene
30. 2-chloro-5-thiophenecarboxylic Acid
31. Act02298
32. Albb-000787
33. Bcp05839
34. Cs-m1254
35. Str03101
36. 5-chloro Thiophen-2-carboxylic Acid
37. 5-chloro-thiophen-2-carboxylic Acid
38. 5-chlorothiophene 2-carboxylic Acid
39. Bbl037515
40. Bdbm50467736
41. Geo-00807
42. Stk502364
43. 5-chloro-2-thiophene-carboxylic Acid
44. 5-chloranylthiophene-2-carboxylic Acid
45. Akos000278212
46. Fs-1378
47. Pb12047
48. Idi1_015104
49. Ac-25302
50. Sy001039
51. 5-chlorothiophen-2-carboxylic Acid
52. 5-chlorothiophene-2-carboxylic Acid, 97%
53. Db-013063
54. A4985
55. Am20090264
56. C1230
57. Ft-0620284
58. Rivaroxaban Impurity F [ep Impurity]
59. W-200249
60. F2182-0126
61. 5-chlorothiophene-2-carboxylic Acid; 2-chloro-5-carboxythiophene; 5-chloro-2-thenoic Acid
62. 9e9
| Molecular Weight | 162.59 g/mol |
|---|---|
| Molecular Formula | C5H3ClO2S |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 161.9542282 g/mol |
| Monoisotopic Mass | 161.9542282 g/mol |
| Topological Polar Surface Area | 65.5 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 128 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


