



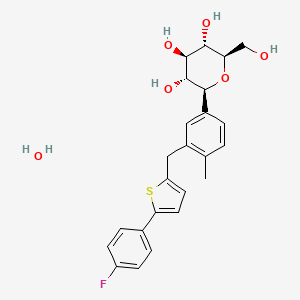

1. 1-(glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene - T777973
2. Canagliflozin
3. Canagliflozin, Anhydrous
4. Invokana
1. Canagliflozin Monohydrate
2. B2l6aes9xq
3. Canagliflozin Hydrate
4. 1809403-05-1
5. D-glucitol, 1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-, Hydrate (1:1), (1s)-
6. Mfcd28975933
7. Unii-b2l6aes9xq
8. Schembl322059
9. Chembl4594217
10. Jnj-28431754; Ta 7284
11. (2s,3r,4r,5s,6r)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol Hemihydrate
| Molecular Weight | 462.5 g/mol |
|---|---|
| Molecular Formula | C24H27FO6S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 462.15123791 g/mol |
| Monoisotopic Mass | 462.15123791 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Sodium-Glucose Transporter 2 Inhibitors
Compounds that inhibit SODIUM-GLUCOSE TRANSPORTER 2. They lower blood sugar by preventing the reabsorption of glucose by the kidney and are used in the treatment of TYPE 2 DIABETES MELLITUS. (See all compounds classified as Sodium-Glucose Transporter 2 Inhibitors.)


