




1. 168828-84-0
2. Deacetamide Linezolid Azide
3. Rk3z2b8jfw
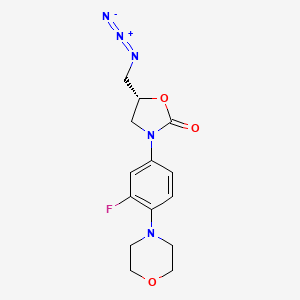
4. (5r)-5-(azidomethyl)-3-(3-fluoro-4-morpholin-4-ylphenyl)-1,3-oxazolidin-2-one
5. (5r)-5-(azidomethyl)-3-(3-fluoro-4-(4-morpholinyl)phenyl)-2-oxazolidinone
6. (r)-(3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-5-oxazolidinyl)methyl Azide
7. (r)-5-azidomethyl-3-(3-fluoro-4-(morpholin-4-yl)phenyl)oxazolidin-2-one
8. (5r)-5-(azidomethyl)-3-[3-fluoro-4-(morpholin-4-yl)phenyl]-1,3-oxazolidin-2-one
9. (r)-(n-(3-fluoro-4-(morpholin-4-yl)phenyl)-2-oxo-5-oxazolidinyl)methyl Azide
10. 2-oxazolidinone, 5-(azidomethyl)-3-(3-fluoro-4-(4-morpholinyl)phenyl)-, (5r)-
11. 2-oxazolidinone, 5-(azidomethyl)-3-(3-fluoro-4-(4-morpholinyl)phenyl)-, (r)-
12. 2-oxazolidinone, 5-(azidomethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-, (5r)-
13. (r)-5-(azidomethyl)-3-(3-fluoro-4-(4-morpholinyl)phenyl)-2-oxazolidinone
14. Linezolide Impurity A
15. Unii-rk3z2b8jfw
16. (r)-5-(azidomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one
17. Chembl5198706
18. Schembl13803066
19. Dtxsid10937555
20. Fqubfddpwlbkiz-nshdsacasa-n
21. Bcp12788
22. Cs-o-31988
23. As-82502
24. A1-03403
25. (5r)-5-(azidomethyl)-3-(3-fluoro-4-morpholinphenyl)oxazolidinone-2- One
26. (5r)-3-(3-fluoro-4-morpholinophenyl)-5alpha-(azidomethyl)oxazolidine-2-one
27. 5-(azidomethyl)-3-[3-fluoro-4-(morpholin-4-yl)phenyl]-1,3-oxazolidin-2-one
28. (r)-5-(azidomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one; Linezolid Related Compound A; Deacetamide Linezolid Azide; Linezolid Related Compound A
| Molecular Weight | 321.31 g/mol |
|---|---|
| Molecular Formula | C14H16FN5O3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 56.4 |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 481 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




