




1. 144702-27-2
2. A8m0gxg1xv
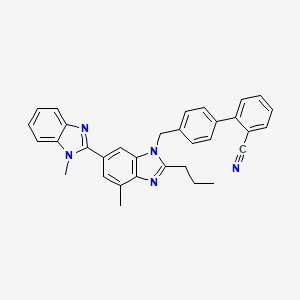
3. 2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propylbenzimidazol-1-yl]methyl]phenyl]benzonitrile
4. (1,1'-biphenyl)-2-carbonitrile, 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1h-benzimidazol)-1'-yl)methyl)-
5. 4'-((4-methyl-6-(1-methyl-1h-benzimidazol-2-yl)-2-propyl-1h-benzimidazol-1-yl)methyl)biphenyl-2-carbonitrile
6. Telmisartan Impurity G
7. Unii-a8m0gxg1xv
8. Telmisartan Impurity G [ep]
9. 4'-[[4-methyl-6-(1-methyl-1h-benzimidazol-2-yl)-2-propyl-1h-benzimidazol-1-yl]methyl]biphenyl-2-carbonitrile
10. Telmisartan Ep Impurity G
11. Schembl2020774
12. Dtxsid20162790
13. Zinc71973211
14. 2-descarboxy-2-cyano Telmisartan-[d3]
15. Db-050162
16. Ft-0665981
17. Telmisartan Impurity G [ep Impurity]
18. Imidazo[1,2-a]pyridine-3-carboxaldehyde,oxime
19. A1-01721
20. Q27273773
21. 4'-(1,4'-dimethyl-2'-propyl-2,6'-bi[1h-benzoimidazole]-1'-ylmethyl)biphenyl-2-carbonitrile
22. 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1h-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carbonitrile
23. 4'-[[2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-1hbenzimidazol-1-yl]-methyl]-2-cyano-biphenyl4'-[[2-n-propyl-4-methyl-6-(1-2methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-2-cyano-biphenyl
24. 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-2-cyano-biphenyl
25. 4?-[[4-methyl-6-(1-methyl-1h-benzimidazol-2-yl)-2-propyl-1h-benzimidazol-1-yl]methyl][1,1?-biphenyl]-2-carbonitrile
| Molecular Weight | 495.6 g/mol |
|---|---|
| Molecular Formula | C33H29N5 |
| XLogP3 | 7.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 495.24229595 g/mol |
| Monoisotopic Mass | 495.24229595 g/mol |
| Topological Polar Surface Area | 59.4 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 829 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






