




1. 1441670-89-8
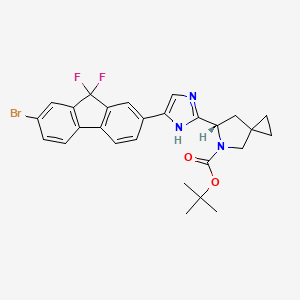
2. Tert-butyl (6s)-6-[5-(7-bromo-9,9-difluorofluoren-2-yl)-1h-imidazol-2-yl]-5-azaspiro[2.4]heptane-5-carboxylate
3. (6s)-6-[5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl]-5-azaspiro[2.4]heptane-5-carboxylic Acid 1,1-dimethylethyl Ester
4. (6s)-tert-butyl-6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro[2.4]heptane-5-carboxylate
5. (s)-tert-butyl 6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro(2.4)heptane-5-carboxylate
6. (s)-tert-butyl6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro[2.4]heptane-5-carboxylate
7. 5-azaspiro[2.4]heptane-5-carboxylic Acid, 6-[5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl]-, 1,1-dimethylethyl Ester, (6s)-
8. Tert-butyl (6s)-6-[5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl]-5-azaspiro[2.4]heptane-5-carboxylate
9. Ec 939-899-8
10. Schembl16541579
11. Schembl19232180
12. Amy4178
13. Dtxsid001101609
14. Cs-z0007
15. Mfcd28386931
16. Akos028109756
17. Zinc141371669
18. Ac-28973
19. Ds-19340
20. Tert-butyl (6s)-6-[5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl]-5-azaspiro[2.4]heptane
21. I11541
22. A853036
23. (6s)-t-butyl-6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro[2.4]heptane-5-carboxylate
24. (s)-tert-butyl 6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro[2.4]heptane-5-carboxylate;ledipasvir Intermediate
25. Tert-butyl (s)-6-(5-(7-bromo-9,9-difluoro-9h-fluoren-2-yl)-1h-imidazol-2-yl)-5-azaspiro[2.4]heptane-5-carboxylate
| Molecular Weight | 542.4 g/mol |
|---|---|
| Molecular Formula | C27H26BrF2N3O2 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 541.11765 g/mol |
| Monoisotopic Mass | 541.11765 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 847 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





