




1. 139481-41-7
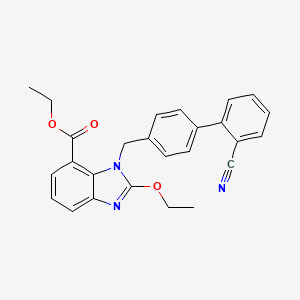
2. Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1h-benzimidazole-7-carboxylate
3. Ethyl-2-ethoxy-1-[[(2'-cyanobiphenyl-4-yl) Methyl] Benzimidazole]-7-carboxylate
4. Ethyl 3-[[4-(2-cyanophenyl)phenyl]methyl]-2-ethoxybenzimidazole-4-carboxylate
5. Ethyl 2-ethoxy-1-[[(2'-cyanobiphenyl-4-yl)methyl]benzimidazole]-7-carboxylate
6. Ethyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-carboxylate
7. Ethyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1h-benzimidazole-7-carboxylate
8. Candesartan Intermediates
9. Ethyl -2-ethoxy-1-[[(2 Inverted Exclamation Marka-cyanobiphenyl-4-yl) Methyl] Benzimidazole] -7-carboxylate
10. Schembl848507
11. Dtxsid80572750
12. Bcp07077
13. Mfcd09030636
14. Zinc21989286
15. Akos015896141
16. Am84368
17. Ds-0460
18. Ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
19. Ac-26326
20. Cs-0151805
21. Ft-0648291
22. 4-chlorophenylphenylphosphoramidochloridate
23. A807543
24. Ethyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole7-carboxylate
25. Ethyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2ethoxybenzimidazole-7-carboxylate
26. Ethyl 1-[(2'-cyano-[1,1'-biphenyl]-4-yl)methyl]-2-ethoxy-1h-benzoimidazole-7-carboxylate
27. Ethyl 1-[(2'-cyano-4-biphenylyl)methyl]-2-ethoxy-1h-benzimidazole-7-carboxylate
28. Ethyl 1-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-2-ethoxy-1h-benzimidazole-7-carboxylate
29. Ethyl 2-ethoxy-1-[[(2`-cyanobiphenyl-4-yl)methyl]benzimidazole]-7-carboxylate
30. Ethyl-2-ethoxy-1-[[(2'-cyanobiphenyl-4-yl)methyl]benzimidazole]-7-carboxylate
31. 1h-benzimidazole-7-carboxylic Acid,1-((2'-cyano(1,1'-biphenyl)-4-yl)methyl)-2-ethoxy-ethylester
32. 1h-benzimidazole-7-carboxylic Acid,1-((2-cyano(1,1-biphenyl)-4-yl)methyl)-2-ethoxy-ethylester
33. 1h-benzimidazole-7-carboxylicacid,1-[(2 Inverted Exclamation Mark -cyano[1,1 Inverted Exclamation Mark -biphenyl]-4-yl)methyl]-2-ethoxy-,ethyl Este
34. Ethyl 1-((2\'-cyano-[1,1\'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
35. Ethyl 1-({2'-cyano-[1,1'-biphenyl]-4-yl}methyl)-2-ethoxy-1h-1,3-benzodiazole-7-carboxylate
36. Ethyl 3-[[4-(2-cyanophenyl)phenyl]methyl]-2-ethoxy-benzimidazole-4-carboxylate;ethyl 1-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
37. Ethyl1-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
| Molecular Weight | 425.5 g/mol |
|---|---|
| Molecular Formula | C26H23N3O3 |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 425.17394160 g/mol |
| Monoisotopic Mass | 425.17394160 g/mol |
| Topological Polar Surface Area | 77.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 669 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




