



1. 106649-96-1
2. 8535iq0676
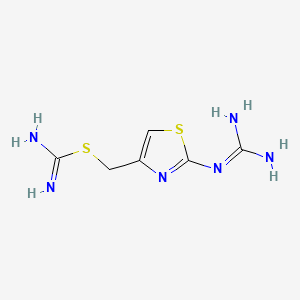
3. [2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methyl Carbamimidothioate
4. Carbamimidothioic Acid, (2-((aminoiminomethyl)amino)-4-thiazolyl)methyl Ester
5. (2-((diaminomethylene)amino)thiazol-4-yl)methyl Carbamimidothioate
6. Famotidine Impurity H [ep]
7. Unii-8535iq0676
8. Starbld0008947
9. Schembl1903834
10. Schembl10782626
11. Dtxsid50147717
12. Zinc2241666
13. Famotidine Impurity H [ep Impurity]
14. S-[(2-guanidino-4-thiazolyl)methyl]isothiourea
15. S-[(2-guanidino-thiazol-4-yl)methyl]isothiourea
16. Q27269585
| Molecular Weight | 230.3 g/mol |
|---|---|
| Molecular Formula | C6H10N6S2 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 230.04083669 g/mol |
| Monoisotopic Mass | 230.04083669 g/mol |
| Topological Polar Surface Area | 181 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 239 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


