

X


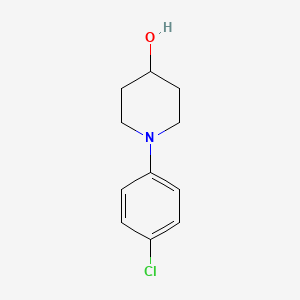

1. 1-(4-chlorophenyl)piperidin-4-ol
2. 119836-12-3
3. Schembl2158833
4. Zinc34415641
5. 4-hydroxy-(4-chlorophenyl)piperidine
6. Akos022304945
7. Db-061660
8. Ft-0710744
| Molecular Weight | 211.69 g/mol |
|---|---|
| Molecular Formula | C11H14ClNO |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 211.0763918 g/mol |
| Monoisotopic Mass | 211.0763918 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 172 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs. 

