16 Jan 2026
// PRESS RELEASE
17 Dec 2025
// PRESS RELEASE
11 Dec 2025
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS KEY EXCIPIENTS
KEY EXCIPIENTS
DKSH is your trusted partner for companies looking to grow their business in Asia & beyond.
About
Industry Trade Show
Not Confirmed
24-26 February, 2026
Industry Trade Show
Not Confirmed
27-29 January, 2026
Industry Trade Show
Not Confirmed
27-28 January, 2026
CONTACT DETAILS




Events
Webinars & Exhibitions
Industry Trade Show
Not Confirmed
24-26 February, 2026
Industry Trade Show
Not Confirmed
27-29 January, 2026
Industry Trade Show
Not Confirmed
27-28 January, 2026
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/fda-approves-record-eight-biosimilars-in-h1-2024-okays-first-interchangeable-biosimilars-for-eylea

16 Jan 2026
// PRESS RELEASE
https://www.dksh.com/in-en/home/investors/investor-news/natale-capri-sole-head-of-business-unit-performance-materials-as-thomas-sul-transitions-into-retirement

17 Dec 2025
// PRESS RELEASE
https://www.dksh.com/in-en/home/investors/investor-news/dksh-technology-signs-biomedic-acquisition-in-vietnam

11 Dec 2025
// PRESS RELEASE
https://mailing-ircockpit.eqs.com/crm-mailing/5be9d1b2-ea7c-11e8-902f-2c44fd856d8c/241a4eb9-d658-4287-9737-0867c625baca/9ca1c6ae-a407-4f23-bf5f-846f08687b30/20251211_Media+Release_Invita.pdf

09 Dec 2025
// PRESS RELEASE
https://mailing-ircockpit.eqs.com/crm-mailing/5be9d1b2-ea7c-11e8-902f-2c44fd856d8c/241a4eb9-d658-4287-9737-0867c625baca/818bceeb-6e96-4e8b-aa0e-f2b3a32bd554/20251209_Media+Release_DHMB.pdf

21 Nov 2025
// PRESS RELEASE
https://www.dksh.com/in-en/home/investors/investor-news/dksh-appoints-patrik-grande-as-new-head-business-unit-healthcare

02 Oct 2025
// PRESS RELEASE
https://mailing-ircockpit.eqs.com/crm-mailing/b6ed963f-ce04-1015-9d5d-b20517257b19/dc35c44f-3ce8-4a5c-b125-6313dbe9b364/a963e751-e7d7-44b1-a3cb-fd8f9a78dce9/DKSH+Media+Release_DKSH+Performance+Materials+Attains+EcoVadis+Gold+Rating+for+its+Sustainability+Achievement.pdf
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
About the Company : DKSH, founded to improve lives, helps businesses expand in existing and emerging markets. With over 155 years of experience, it operates in 36 markets, is listed on the SIX Swiss Exchange, emplo...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Details:
DKSH will undertake the responsibility for providing comprehensive Market Expansion Services for triglycerides lowering drug, Parmodia (pemafibrate).
Lead Product(s): Pemafibrate
Therapeutic Area: Endocrinology Brand Name: Parmodia
Study Phase: Approved FDFProduct Type: Miscellaneous
Recipient: Kowa Pharmaceuticals America
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership January 24, 2025
Lead Product(s) : Pemafibrate
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Recipient : Kowa Pharmaceuticals America
Deal Size : Undisclosed
Deal Type : Partnership
Kowa and DKSH Debut Triglycerides Lowering Medicine, Parmodia®, in Malaysia
Details : DKSH will undertake the responsibility for providing comprehensive Market Expansion Services for triglycerides lowering drug, Parmodia (pemafibrate).
Product Name : Parmodia
Product Type : Miscellaneous
Upfront Cash : Undisclosed
January 24, 2025
Details:
DKSH will support the commercialization of human papillomavirus (HPV) vaccines, including Gardasil, in Thailand.
Lead Product(s): HPV Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant
Therapeutic Area: Infections and Infectious Diseases Brand Name: Gardasil
Study Phase: Approved FDFProduct Type: Vaccine
Recipient: Merck & Co
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership December 12, 2024
Lead Product(s) : HPV Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Recipient : Merck & Co
Deal Size : Undisclosed
Deal Type : Partnership
DKSH Supports MSD’s Expansion with New Partnership to Improve Vaccine Access in Thailand
Details : DKSH will support the commercialization of human papillomavirus (HPV) vaccines, including Gardasil, in Thailand.
Product Name : Gardasil
Product Type : Vaccine
Upfront Cash : Undisclosed
December 12, 2024
Details:
The partnership covers a promotion and distribution agreement for commercial rights of Kyowa’s established medicines, including Crysvita (burosumab), indicated for X-linked hypophosphatemia.
Lead Product(s): Burosumab
Therapeutic Area: Rare Diseases and Disorders Brand Name: Crysvita
Study Phase: Approved FDFProduct Type: Antibody, Unconjugated
Recipient: Kyowa Kirin
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership August 02, 2024
Lead Product(s) : Burosumab
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Approved FDF
Recipient : Kyowa Kirin
Deal Size : Undisclosed
Deal Type : Partnership
DKSH and Kyowa Kirin Forge Strategic Partnership Across Asia-Pacific
Details : The partnership covers a promotion and distribution agreement for commercial rights of Kyowa’s established medicines, including Crysvita (burosumab), indicated for X-linked hypophosphatemia.
Product Name : Crysvita
Product Type : Antibody, Unconjugated
Upfront Cash : Undisclosed
August 02, 2024
Details:
DKSH has enetered a strategic partnership with SciGen to supply Zometa (zoledronic acid) to the Vietnamese market. it is indicated for the treatment of Advanced cancers.
Lead Product(s): Zoledronic Acid
Therapeutic Area: Oncology Brand Name: Zometa
Study Phase: Approved FDFProduct Type: Miscellaneous
Recipient: SciGen
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership July 21, 2023
Lead Product(s) : Zoledronic Acid
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Recipient : SciGen
Deal Size : Undisclosed
Deal Type : Partnership
DKSH Has Successfully Entered into a Strategic Partnership with SciGen in Vietnam
Details : DKSH has enetered a strategic partnership with SciGen to supply Zometa (zoledronic acid) to the Vietnamese market. it is indicated for the treatment of Advanced cancers.
Product Name : Zometa
Product Type : Miscellaneous
Upfront Cash : Undisclosed
July 21, 2023
Details:
Under the cooperation agreement, DKSH HK will provide full-agency services including key account management, marketing promotion, supply chain, and distribution for Bentrio (bentonite) Nasal Spray sales operation in Hong Kong and Macau.
Lead Product(s): Bentonite,Citric Acid,Propylene Glycol
Therapeutic Area: Immunology Brand Name: Bentrio
Study Phase: Approved FDFProduct Type: Miscellaneous
Recipient: Nuance Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership November 28, 2022
Lead Product(s) : Bentonite,Citric Acid,Propylene Glycol
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Recipient : Nuance Pharma
Deal Size : Undisclosed
Deal Type : Partnership
Nuance Pharma Announces Partnership With DKSH To Launch Bentrio™ Nasal Spray In Hong Kong And Ma...
Details : Under the cooperation agreement, DKSH HK will provide full-agency services including key account management, marketing promotion, supply chain, and distribution for Bentrio (bentonite) Nasal Spray sales operation in Hong Kong and Macau.
Product Name : Bentrio
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 28, 2022
Details:
Myonal (eperisone hydrochloride) is a muscle-relaxant which is used to treat neck-shoulder-arm syndrome and Merislon is approved to treat vertigo and dizziness associated with inner-ear disorders.
Lead Product(s): Eperisone Hydrochloride
Therapeutic Area: Neurology Brand Name: Myonal
Study Phase: Approved FDFProduct Type: Miscellaneous
Recipient: Eisai
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement November 07, 2022
Lead Product(s) : Eperisone Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Recipient : Eisai
Deal Size : Undisclosed
Deal Type : Agreement
Eisai To Divest Rights For Muscle Relaxant Myonal® And Vertigo And Equilibrium Disturbance Treatm...
Details : Myonal (eperisone hydrochloride) is a muscle-relaxant which is used to treat neck-shoulder-arm syndrome and Merislon is approved to treat vertigo and dizziness associated with inner-ear disorders.
Product Name : Myonal
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 07, 2022
Details:
The agreement will support both LEO Pharma’s strategy of building a simpler and more competitive organization and pipeline including its lead asset LP0133 (delgocitinib) and DKSH Business Unit Healthcare’s strategic focus of strengthening its regional footprint.
Lead Product(s): Delgocitinib
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Recipient: Leo Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership September 29, 2022
Lead Product(s) : Delgocitinib
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Recipient : Leo Pharma
Deal Size : Undisclosed
Deal Type : Partnership
Details : The agreement will support both LEO Pharma’s strategy of building a simpler and more competitive organization and pipeline including its lead asset LP0133 (delgocitinib) and DKSH Business Unit Healthcare’s strategic focus of strengthening its regiona...
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
September 29, 2022
Details:
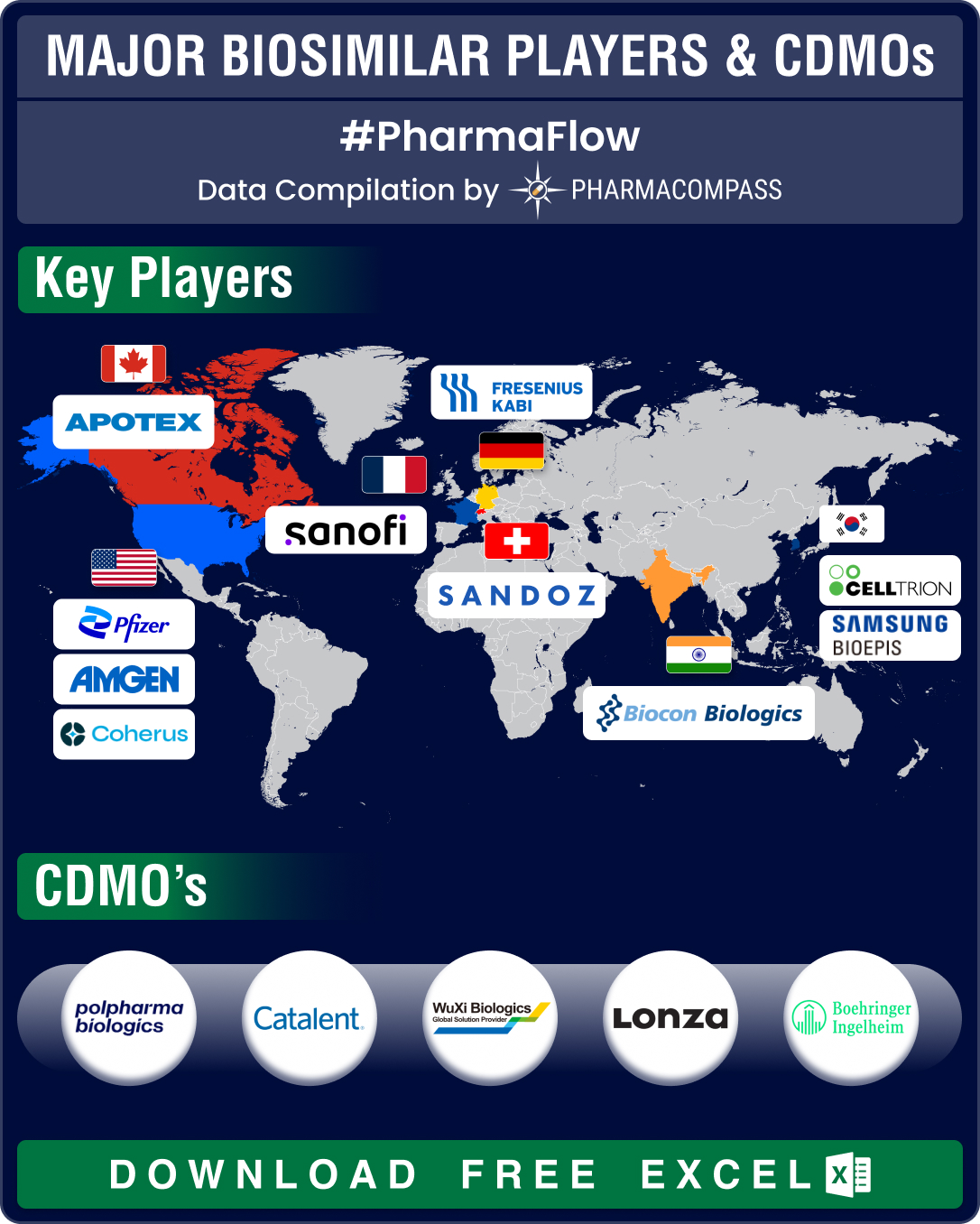
The biosimilars planned under this agreement include biosimilar of Prolia® (denosumab, AVT03), Xgeva® (denosumab), Simponi® (golimumab), and Eylea® (aflibercept) as well as two undisclosed proposed biosimilars for immunology and oncology.
Lead Product(s): Denosumab
Therapeutic Area: Musculoskeletal Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Antibody, Unconjugated
Recipient: Lupin Ltd
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement September 07, 2022
Lead Product(s) : Denosumab
Therapeutic Area : Musculoskeletal
Highest Development Status : Phase III
Recipient : Lupin Ltd
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Details : The biosimilars planned under this agreement include biosimilar of Prolia® (denosumab, AVT03), Xgeva® (denosumab), Simponi® (golimumab), and Eylea® (aflibercept) as well as two undisclosed proposed biosimilars for immunology and oncology.
Product Name : Undisclosed
Product Type : Antibody, Unconjugated
Upfront Cash : Undisclosed
September 07, 2022
Details:
Under the partnership, Alvotech Hf will be responsible for the development and supply of AVT02, while DKSH will oversee its registration and commercialization.
Lead Product(s): Adalimumab
Therapeutic Area: Immunology Brand Name: Simlandi
Study Phase: Phase IIIProduct Type: Antibody, Unconjugated
Recipient: Alvotech
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership March 24, 2020
Lead Product(s) : Adalimumab
Therapeutic Area : Immunology
Highest Development Status : Phase III
Recipient : Alvotech
Deal Size : Undisclosed
Deal Type : Partnership
Alvotech and DKSH partner to bring key biosimilar to Asia
Details : Under the partnership, Alvotech Hf will be responsible for the development and supply of AVT02, while DKSH will oversee its registration and commercialization.
Product Name : Simlandi
Product Type : Antibody, Unconjugated
Upfront Cash : Undisclosed
March 24, 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients
Excipients by Ingredients
Excipients By applications
ABOUT THIS PAGE
DKSH is a supplier offers 119 products (APIs, Excipients or Intermediates).
Find a price of Acemetacin bulk offered by DKSH
Find a price of Ajmaline bulk offered by DKSH
Find a price of Alpha Lipoic Acid bulk offered by DKSH
Find a price of Aluminium Hydroxide bulk offered by DKSH
Find a price of Amiodarone Hydrochloride bulk offered by DKSH
Find a price of Apixaban bulk offered by DKSH
Find a price of Artemether bulk offered by DKSH
Find a price of Aspirin bulk offered by DKSH
Find a price of Atorvastatin bulk offered by DKSH
Find a price of Atracurium Besylate bulk offered by DKSH
Find a price of Avermectin bulk offered by DKSH
Find a price of Azacitidine bulk offered by DKSH
Find a price of Azathioprine bulk offered by DKSH
Find a price of Azithromycin bulk offered by DKSH
Find a price of Baloxavir Marboxil bulk offered by DKSH
Find a price of Bortezomib bulk offered by DKSH
Find a price of Bromhexine Hydrochloride bulk offered by DKSH
Find a price of Budesonide bulk offered by DKSH
Find a price of Carbamazepine bulk offered by DKSH
Find a price of Carfilzomib bulk offered by DKSH
Find a price of Carvedilol bulk offered by DKSH
Find a price of Caspofungin bulk offered by DKSH
Find a price of Chlorhexidine Gluconate bulk offered by DKSH
Find a price of Cimetropium Bromide bulk offered by DKSH
Find a price of Citicoline Sodium bulk offered by DKSH
Find a price of Clarithromycin bulk offered by DKSH
Find a price of Colchicine bulk offered by DKSH
Find a price of Deferasirox bulk offered by DKSH
Find a price of Desloratadine bulk offered by DKSH
Find a price of Dexamethasone bulk offered by DKSH
Find a price of Diclofenac Sodium bulk offered by DKSH
Find a price of Dienogest bulk offered by DKSH
Find a price of Digoxin bulk offered by DKSH
Find a price of Diisopropylamine Dichloroacetate bulk offered by DKSH
Find a price of Diphenhydramine Hydrochloride bulk offered by DKSH
Find a price of Doxazosin Mesylate bulk offered by DKSH
Find a price of Doxorubicin Hydrochloride bulk offered by DKSH
Find a price of Doxycycline bulk offered by DKSH
Find a price of Doxycycline Hyclate bulk offered by DKSH
Find a price of Drospirenone bulk offered by DKSH
Find a price of Emtricitabine bulk offered by DKSH
Find a price of Epirubicin Hydrochloride bulk offered by DKSH
Find a price of Erythromycin bulk offered by DKSH
Find a price of Everolimus bulk offered by DKSH
Find a price of Ezetimibe bulk offered by DKSH
Find a price of Fluocinolone Acetonide bulk offered by DKSH
Find a price of Gabapentin bulk offered by DKSH
Find a price of Guaifenesin bulk offered by DKSH
Find a price of Haloperidol bulk offered by DKSH
Find a price of Icosapent Ethyl bulk offered by DKSH
Find a price of Indacaterol Maleate bulk offered by DKSH
Find a price of Indapamide bulk offered by DKSH
Find a price of Itraconazole bulk offered by DKSH
Find a price of Ivermectin bulk offered by DKSH
Find a price of Ketotifen Fumarate bulk offered by DKSH
Find a price of Lamivudine bulk offered by DKSH
Find a price of Lamotrigine bulk offered by DKSH
Find a price of Levocetirizine Dihydrochloride bulk offered by DKSH
Find a price of Levofloxacin bulk offered by DKSH
Find a price of Levofloxacin Hemihydrate bulk offered by DKSH
Find a price of Lidamidine Hydrochloride bulk offered by DKSH
Find a price of Lidocaine bulk offered by DKSH
Find a price of Loratadine bulk offered by DKSH
Find a price of Magaldrate bulk offered by DKSH
Find a price of Magnesium Hydroxide bulk offered by DKSH
Find a price of Mesalazine bulk offered by DKSH
Find a price of Metaxalone bulk offered by DKSH
Find a price of Methocarbamol bulk offered by DKSH
Find a price of Methscopolamine Bromide bulk offered by DKSH
Find a price of Methyl Salicylate bulk offered by DKSH
Find a price of Metronidazole bulk offered by DKSH
Find a price of Miconazole Nitrate bulk offered by DKSH
Find a price of Mometasone Furoate bulk offered by DKSH
Find a price of Mosapride Citrate bulk offered by DKSH
Find a price of Moxifloxacin Hydrochloride bulk offered by DKSH
Find a price of Mycophenolate Mofetil bulk offered by DKSH
Find a price of Mycophenolate Mofetil Hydrochloride bulk offered by DKSH
Find a price of Neomycin Sulfate bulk offered by DKSH
Find a price of Nicorandil bulk offered by DKSH
Find a price of Noscapine bulk offered by DKSH
Find a price of Noscapine Hydrochloride bulk offered by DKSH
Find a price of Olanzapine bulk offered by DKSH
Find a price of Omega-3-Carboxylic Acids bulk offered by DKSH
Find a price of Omeprazole bulk offered by DKSH
Find a price of Paclitaxel bulk offered by DKSH
Find a price of Pantoprazole Sodium bulk offered by DKSH
Find a price of Papaverine Hydrochloride bulk offered by DKSH
Find a price of Paracetamol bulk offered by DKSH
Find a price of Paroxetine Hydrochloride bulk offered by DKSH
Find a price of Pentosan Polysulfate Sodium bulk offered by DKSH
Find a price of Pheniramine Maleate bulk offered by DKSH
Find a price of Pranlukast Hydrate bulk offered by DKSH
Find a price of Prednisone bulk offered by DKSH
Find a price of Procaine bulk offered by DKSH
Find a price of Quetiapine Hemifumarate bulk offered by DKSH
Find a price of Rabeprazole Sodium bulk offered by DKSH
Find a price of Racecadotril bulk offered by DKSH
Find a price of Reserpine bulk offered by DKSH
Find a price of Rosuvastatin Calcium bulk offered by DKSH
Find a price of Ruxolitinib Phosphate bulk offered by DKSH
Find a price of Salbutamol Sulphate bulk offered by DKSH
Find a price of Scopolamine bulk offered by DKSH
Find a price of Scopolamine Hydrobromide bulk offered by DKSH
Find a price of Sirolimus bulk offered by DKSH
Find a price of Sugammadex Sodium bulk offered by DKSH
Find a price of Tacrolimus bulk offered by DKSH
Find a price of Tadalafil bulk offered by DKSH
Find a price of Tamsulosin bulk offered by DKSH
Find a price of Telmisartan bulk offered by DKSH
Find a price of Tenofovir Alafenamide Fumarate bulk offered by DKSH
Find a price of Tenofovir Disoproxil Fumarate bulk offered by DKSH
Find a price of Tenoxicam bulk offered by DKSH
Find a price of Terbinafine bulk offered by DKSH
Find a price of Terbinafine Hydrochloride bulk offered by DKSH
Find a price of Thiocolchicoside bulk offered by DKSH
Find a price of Ulipristal Acetate bulk offered by DKSH
Find a price of Vildagliptin bulk offered by DKSH
Find a price of Vonoprazan Fumarate bulk offered by DKSH
Find a price of Zoledronic Acid bulk offered by DKSH


 DKSH
DKSH