NDC Code(s) : 70377-038-11
Packager : Biocon Phama Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| POSACONAZOLEPosaconazole TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Biocon Phama Inc.(080000063) |
| REGISTRANT - Biocon Pharma Limited(871412155) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Biocon Pharma Limited | 871412155 | analysis(70377-038), manufacture(70377-038), pack(70377-038) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Biocon Limited | 915076162 | api manufacture(70377-038) | |
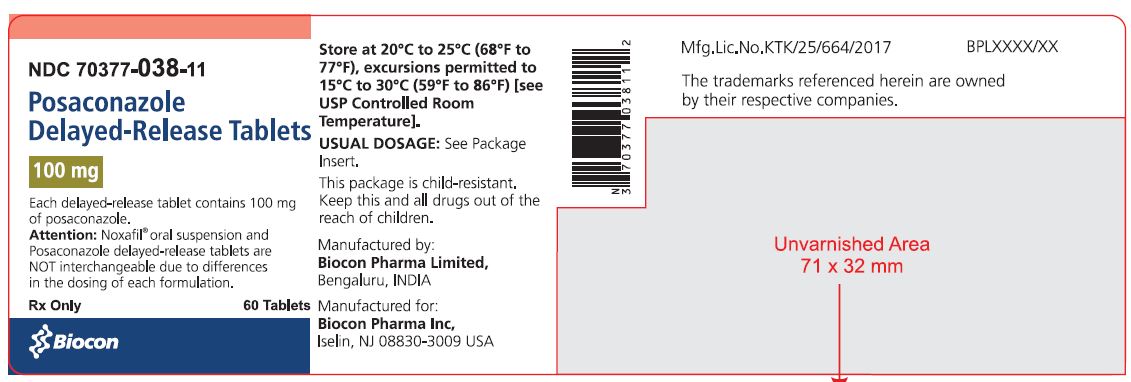
PRINCIPAL DISPLAY PANEL
NDC 70377-038-11
Posaconazole Delayed-Release Tablets
100 mg
Attention: Noxafil® Oral Suspension and Posaconazole Delayed-
Release Tablets are NOT interchangeable due to
differences in the dosing of each formulation.
Rx only 60 Tablets