NDC Code(s) : 70069-005-01, 70069-005-10, 70069-172-01, 70069-172-10, 70069-172-25
Packager : Somerset Therapeutics, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| cyanocobalamincyanocobalamin INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| cyanocobalamincyanocobalamin INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Somerset Therapeutics, LLC(079947873) |
| REGISTRANT - Somerset Therapeutics, LLC(079947873) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Somerset Therapeutics Limited | 677236695 | ANALYSIS(70069-005, 70069-172), LABEL(70069-005, 70069-172), MANUFACTURE(70069-005, 70069-172), PACK(70069-005, 70069-172) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Maiva Pharma Private Limited | 725656438 | ANALYSIS(70069-005), LABEL(70069-005), MANUFACTURE(70069-005), PACK(70069-005) | |
PRINCIPAL DISPLAY PANEL
Cyanocobalamin Injection, USP
1 mL Container Label
NDC 70069-005-01
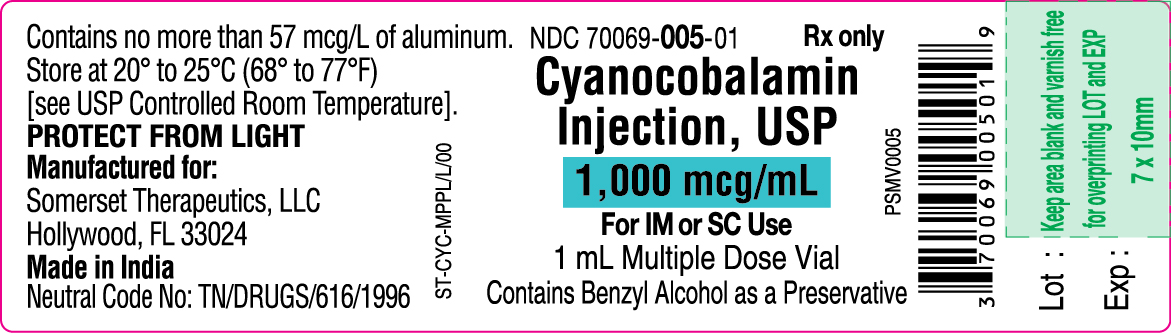
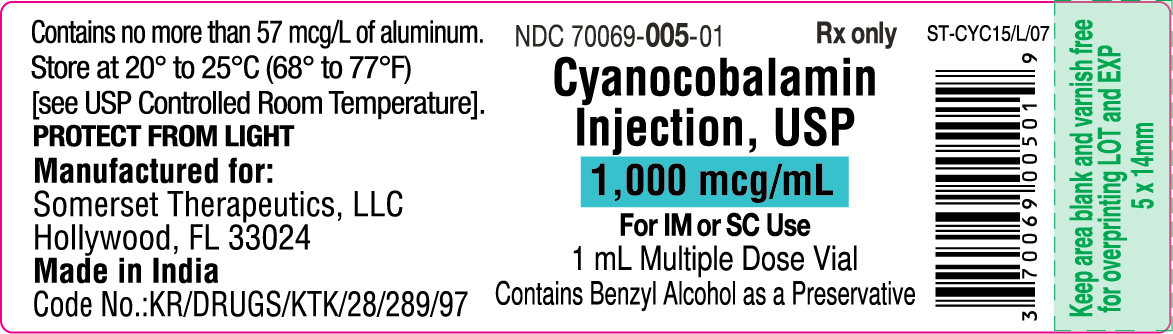 Container Label
Container Label
Cyanocobalamin Injection, USP
1 mL Carton Label
NDC 70069-005-10
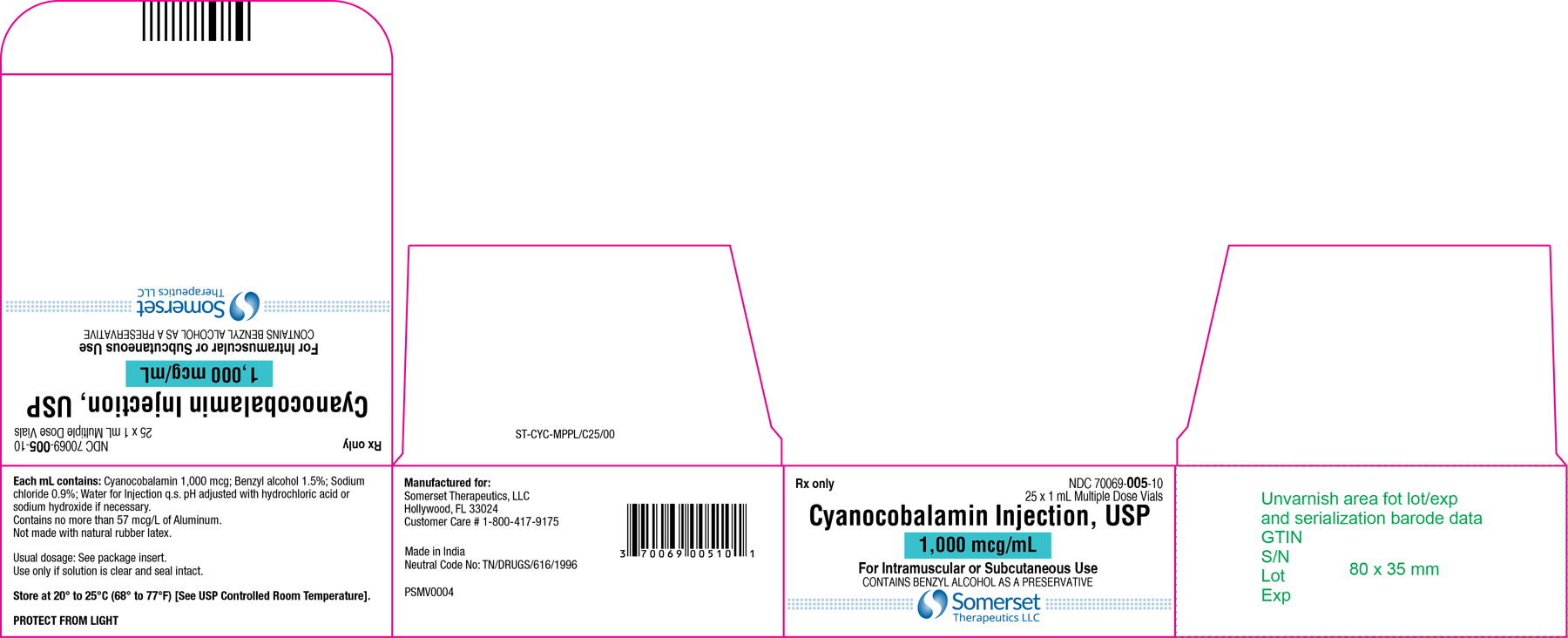
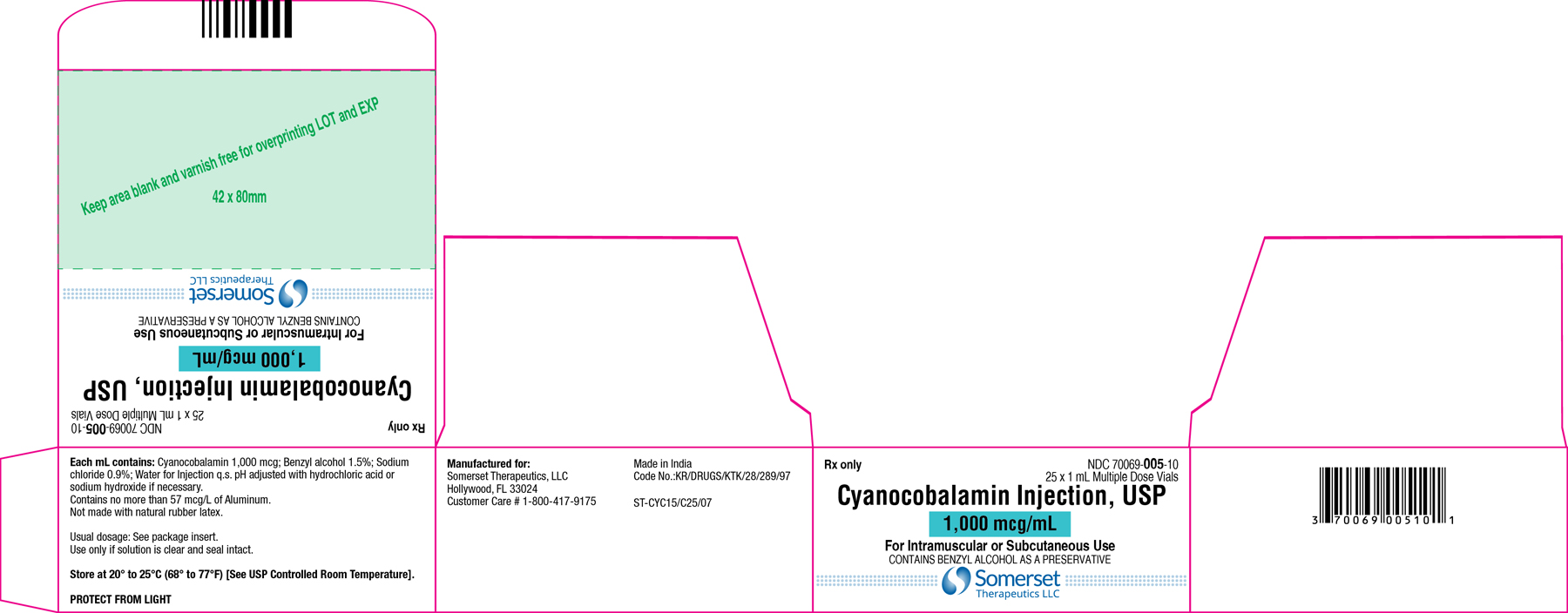 Carton Label
Carton Label
Cyanocobalamin Injection, USP
10 mL Container Label
NDC 70069-172-01
 container Label
container Label
Cyanocobalamin Injection, USP
10 mL Carton Label – Pack of 25's
NDC 70069-172-25
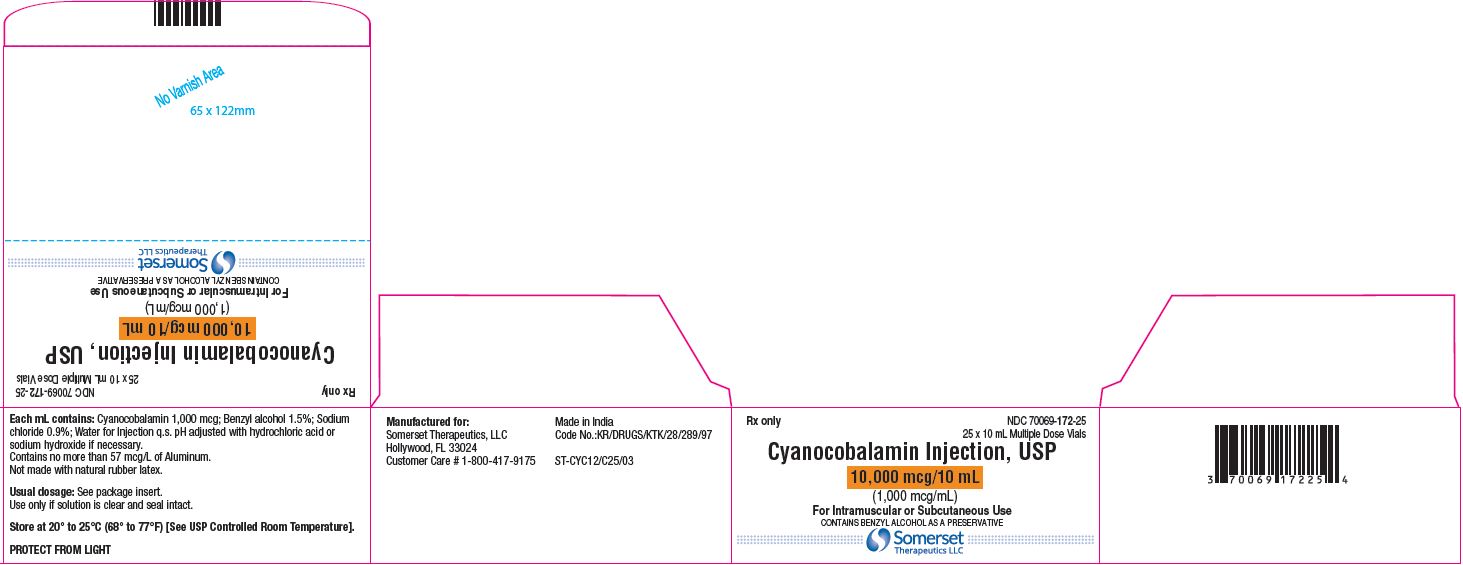 carton label
carton label
Cyanocobalamin Injection, USP
10 mL Carton Label – Pack of 10's
NDC 70069-172-10
 carton-label
carton-label








