NDC Code(s) : 69097-363-44
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TestosteroneTestosterone SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Ltd.- Goa | 650072015 | MANUFACTURE(69097-363) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aspen Oss B.V | 491013870 | ANALYSIS(69097-363), API MANUFACTURE(69097-363), PACK(69097-363), STERILIZE(69097-363) | |
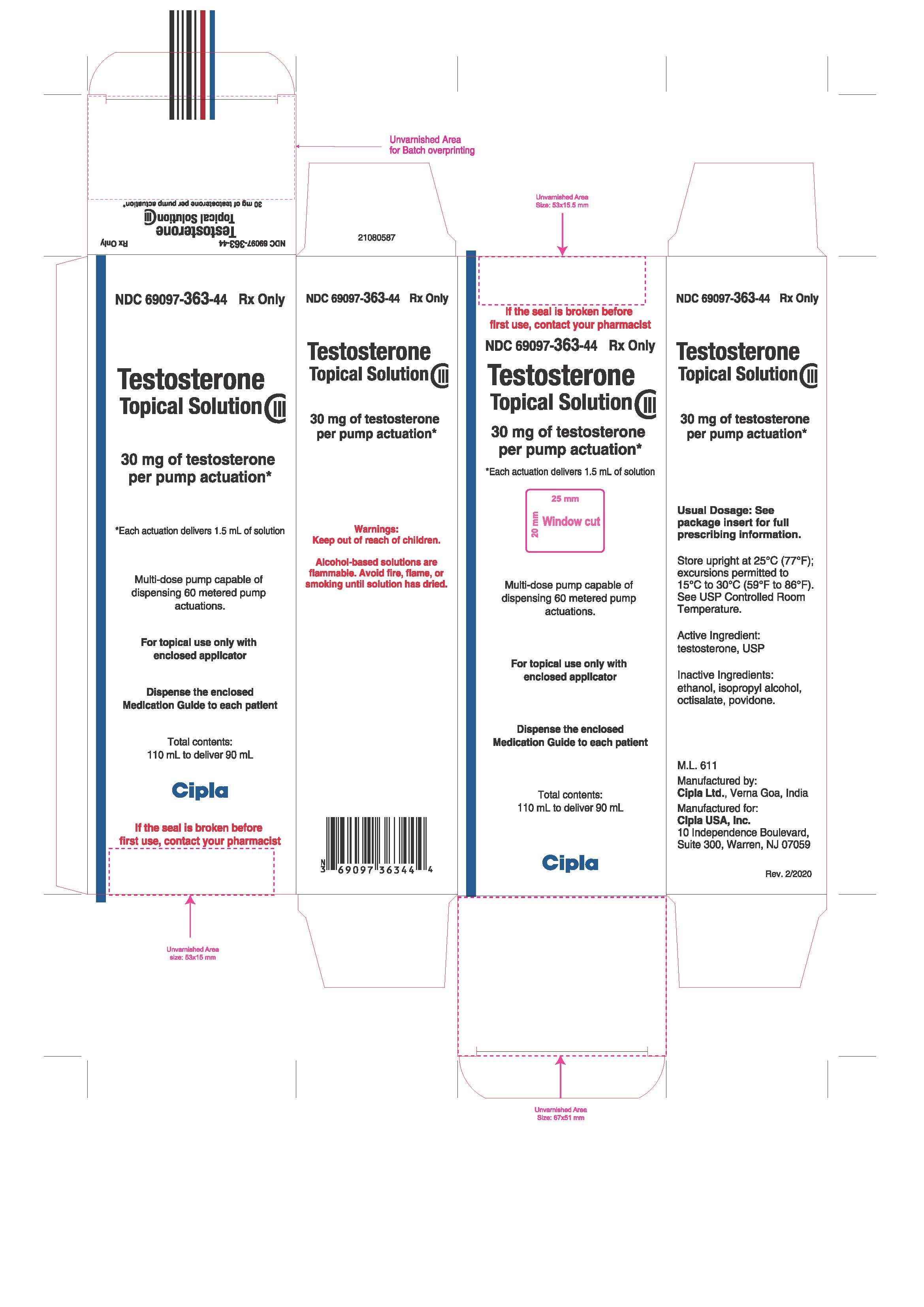
PRINCIPAL DISPLAY PANEL
NDC 69097-363-44
Rx ONLY
Testosterone
Topical Solution
CIII
30 mg of testosterone
per pump actuation*
*Each actuation delivers 1.5 mL of solution
Total contents:
110 mL to deliver 90 mL
 image
image
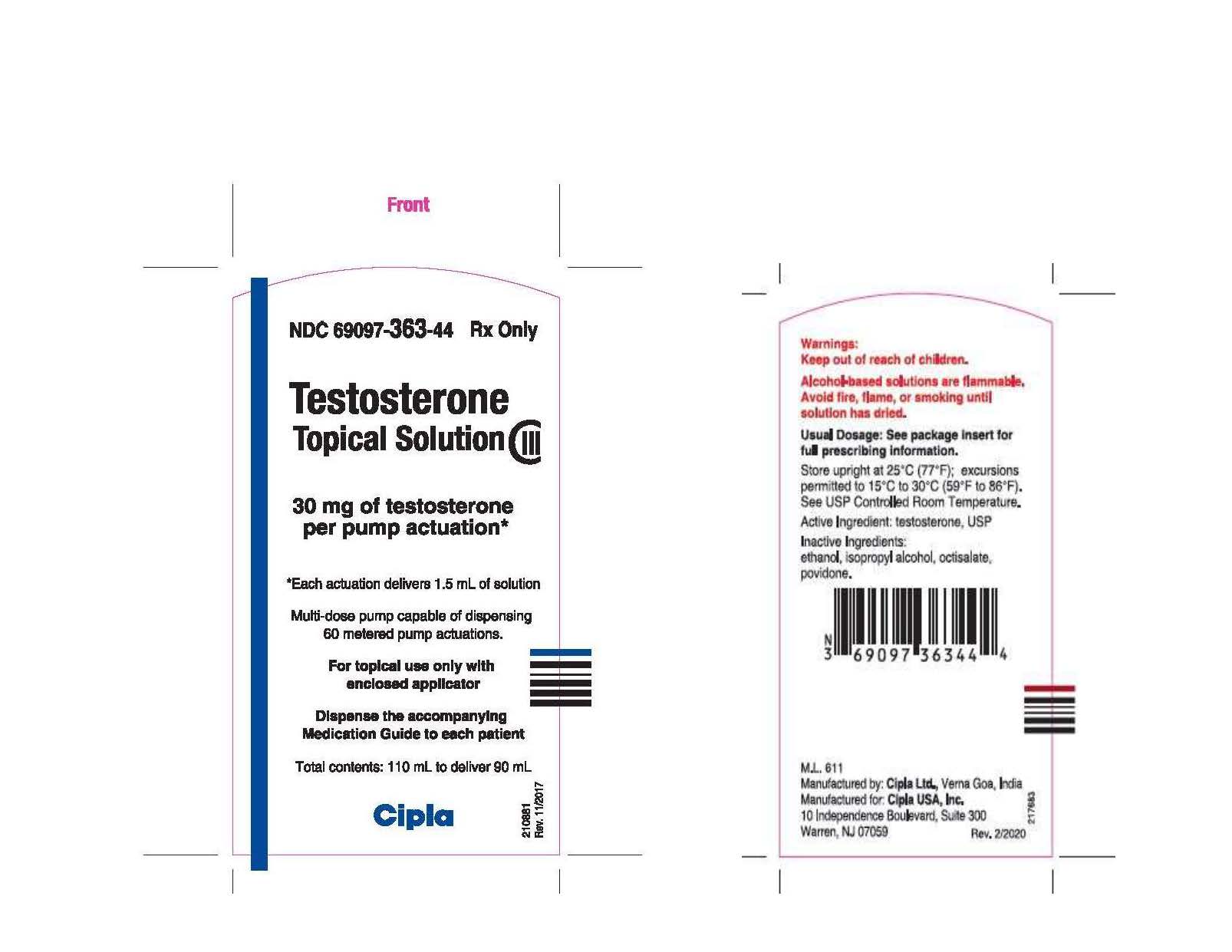
NDC 69097-363-44
Rx ONLY
Testosterone
Topical Solution
CIII
30 mg of testosterone
per pump actuation*
*Each actuation delivers 1.5 mL of solution
Total contents:
110 mL to deliver 90 mL
 image
image








