NDC Code(s) : 68788-8520-6
Packager : Preferred Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Metformin HydrochlorideMetformin Hydrochloride TABLET, EXTENDED RELEASE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Preferred Pharmaceuticals, Inc.(791119022) |
| REGISTRANT - Preferred Pharmaceuticals, Inc.(791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Preferred Pharmaceuticals, Inc. | 791119022 | RELABEL(68788-8520) | |
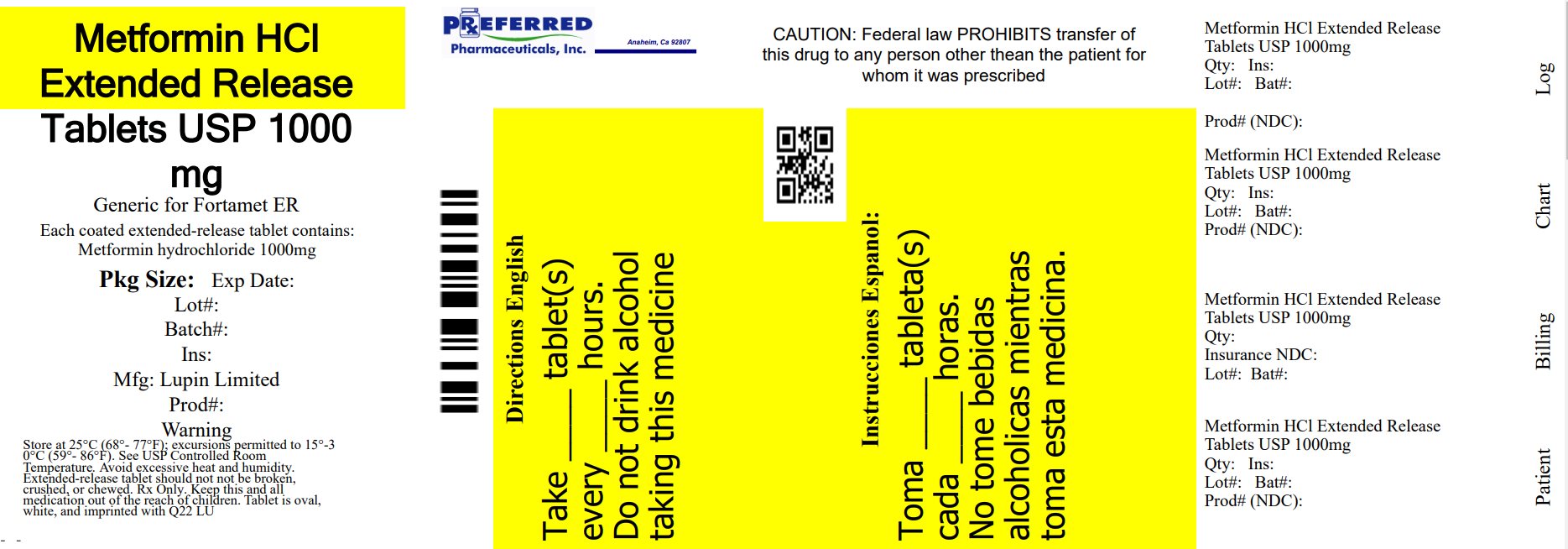
PRINCIPAL DISPLAY PANEL
Metformin Hydrochloride Extended-Release Tablets.
Rx only
1000 mg
NDC 68788-8520-6
60 Tablet Bottles