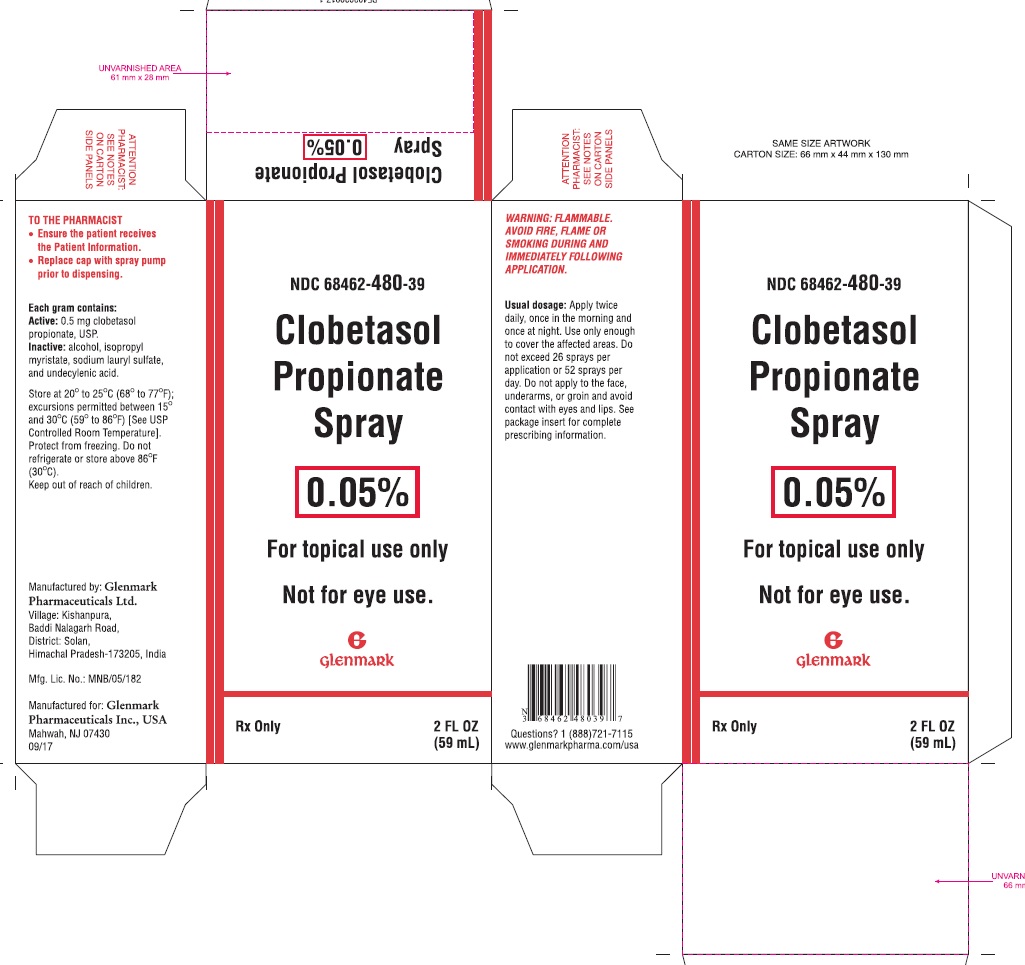
NDC Code(s) : 68462-480-39, 68462-480-56
Packager : Glenmark Pharmaceuticals Inc., USA
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLOBETASOL PROPIONATECLOBETASOL PROPIONATE SPRAY | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Glenmark Pharmaceuticals Inc., USA(130597813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Glenmark Pharmaceuticals Limited | 676115028 | ANALYSIS(68462-480), MANUFACTURE(68462-480) | |
PRINCIPAL DISPLAY PANEL
NDC 68462-480-39
Clobetasol Propionate Spray, 0.05%
For topical use only. Not for eye use.