NDC Code(s) : 68462-386-30, 68462-386-90, 68462-386-14, 68462-387-30, 68462-387-90, 68462-387-14
Packager : Glenmark Pharmaceuticals Inc., USA
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| solifenacin succinatesolifenacin succinate TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| solifenacin succinatesolifenacin succinate TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Glenmark Pharmaceuticals Inc., USA(130597813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Glenmark Pharmaceuticals Limited | 862603186 | MANUFACTURE(68462-386, 68462-387), ANALYSIS(68462-386, 68462-387) | |
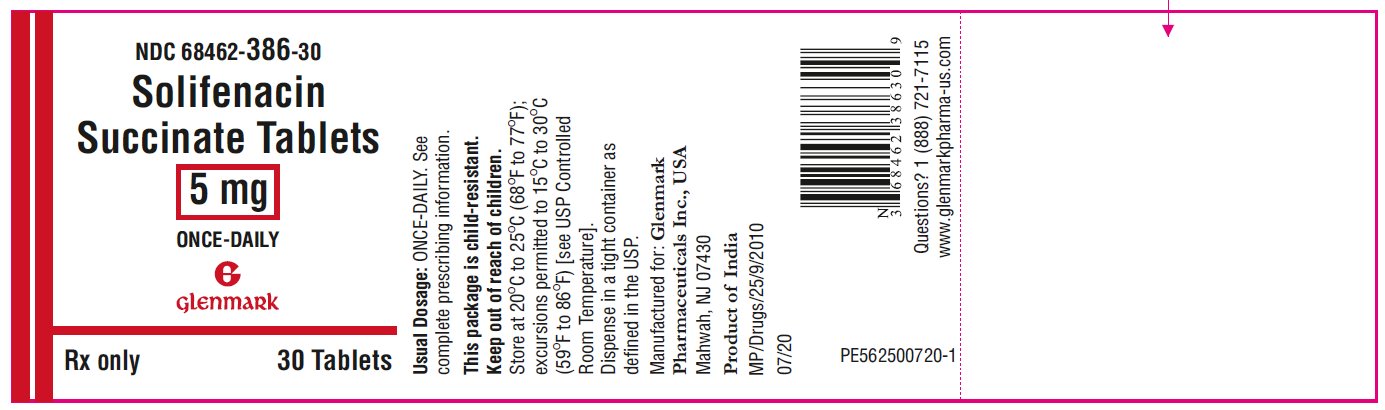
PRINCIPAL DISPLAY PANEL
NDC 68462-386-30
Solifenacin Succinate Tablets, 5 mg
30 Tablets-Bottle
PRINCIPAL DISPLAY PANEL
NDC 68462-387-30
Solifenacin Succinate Tablets, 10 mg
30 Tablets-Bottle









