NDC Code(s) : 68001-524-28, 68001-524-30, 68001-524-29, 68001-524-31
Packager : BluePoint Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FluorouracilFluorouracil INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - BluePoint Laboratories(985523874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Gland Pharma Limited | 650540227 | analysis(68001-524), manufacture(68001-524), pack(68001-524) | |
PRINCIPAL DISPLAY PANEL
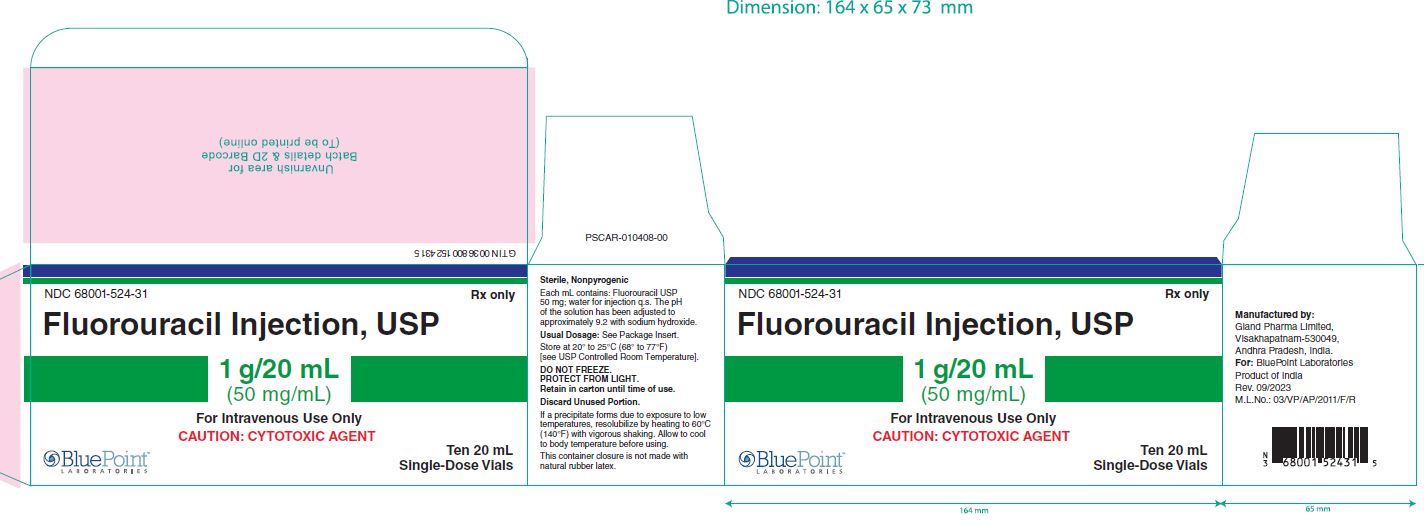
Fluorouracil Injection, USP
NDC 68001-524-28
Container Label-10mL
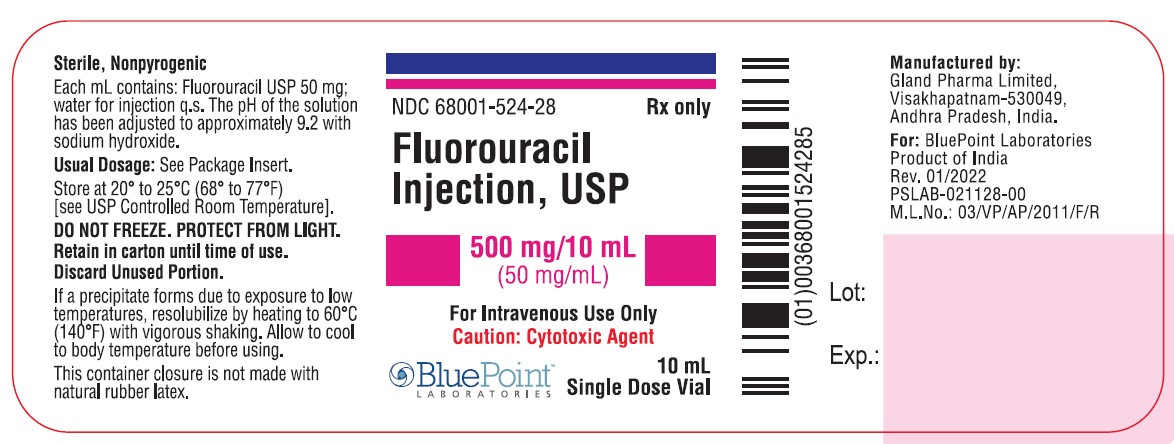
Fluorouracil Injection, USP
NDC 68001-524-30
Carton Label - 10mL
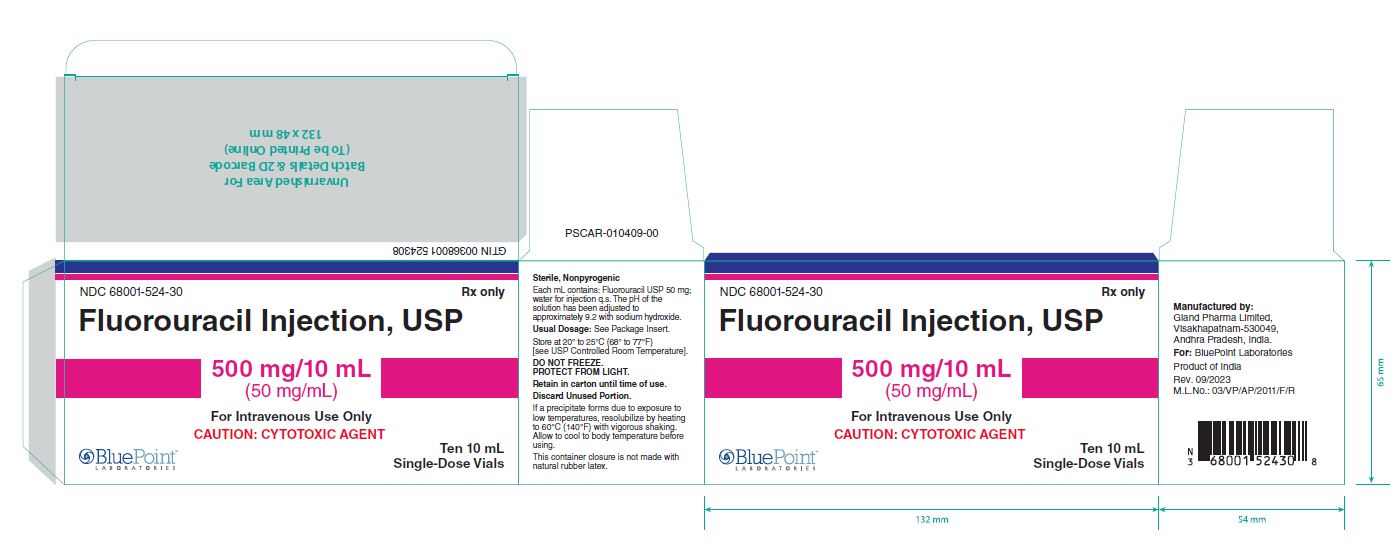
Fluorouracil Injection, USP
NDC 68001-524-29
Container Label-20mL
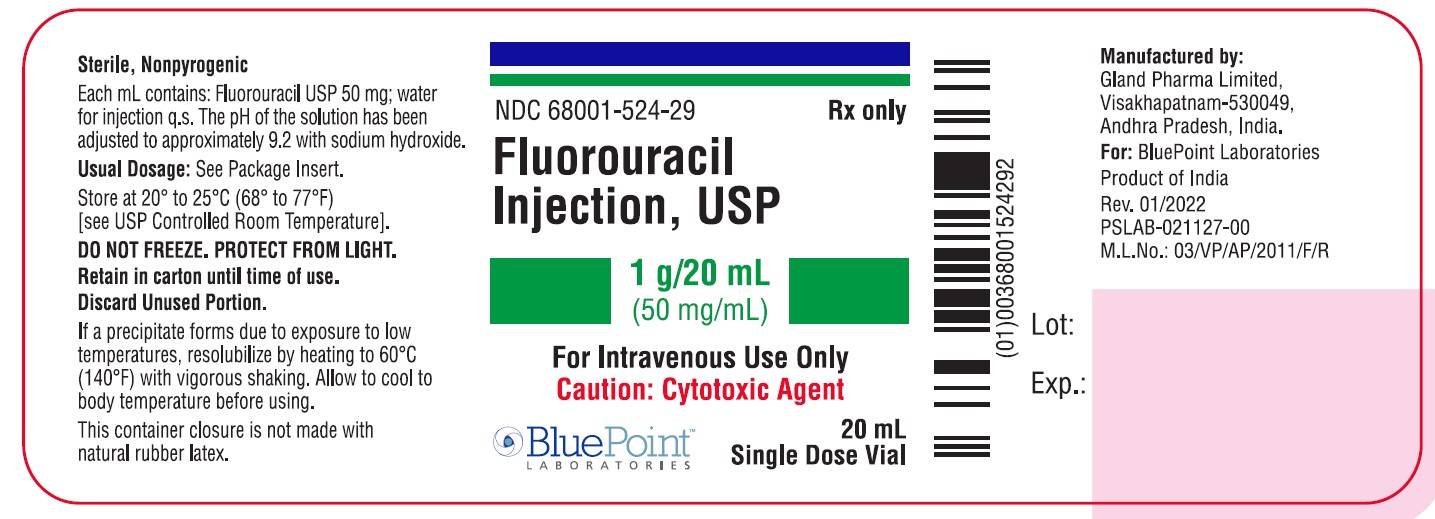
Fluorouracil Injection, USP
NDC 68001-524-31
Carton Label - 20mL