NDC Code(s) : 63323-871-15
Packager : Fresenius Kabi USA, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DaptomycinDaptomycin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Fresenius Kabi USA, LLC(608775388) |
| REGISTRANT - Xellia Pharmaceuticals ApS(305814345) |
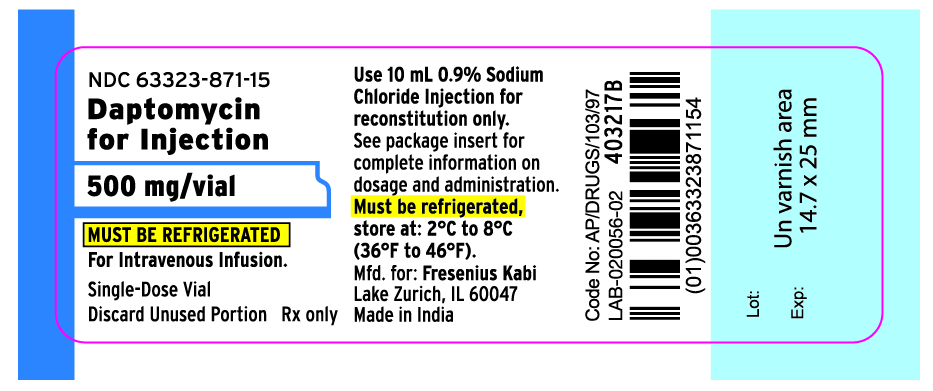
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 500 mg Vial Label
NDC 63323-871-15
Daptomycin for Injection
500 mg/vial
Must be refrigerated.
For Intravenous Infusion.
Single-Dose Vial
Discard Unused Portion Rx only
PRINCIPAL DISPLAY PANEL - 500 mg Vial Carton
NDC 63323-871-15
Daptomycin for Injection
500 mg/vial
Must be refrigerated.
For Intravenous Infusion.
Use 0.9% Sodium Chloride Injection for reconstitution only.
One Single-Dose Vial
Discard Unused Portion
Rx only FRESENIUS KABI logo









