NDC Code(s) : 58602-765-76
Packager : Aurohealth LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Childrens Pain ReliefAcetaminophen TABLET, CHEWABLE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - Aurohealth LLC(078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| APL HEALTHCARE LIMITED | 650844777 | manufacture(58602-765) | |
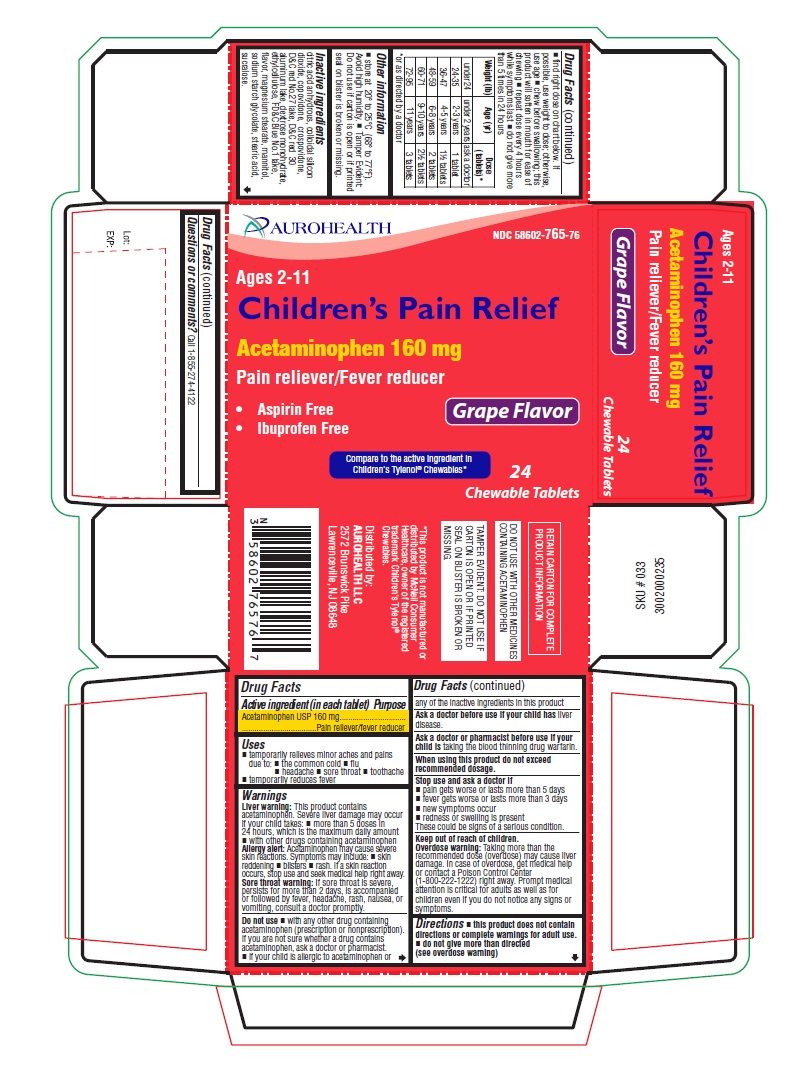
PRINCIPAL DISPLAY PANEL
AUROHEALTH
NDC 58602-765-76
Ages 2-11
Children's Pain Relief
Acetaminophen 160 mg
Pain reliever/Fever reducer
- Aspirin Free
- Ibuprofen Free
Grape Flavor
Compare to the active ingredient in
Children's Tylenol® Chewables*
24
Chewable Tablets