NDC Code(s) : 58118-0519-9, 58118-0519-3, 58118-0519-8, 58118-0519-0, 58118-1520-9, 58118-1520-3, 58118-1520-8, 58118-1518-9, 58118-1518-3, 58118-1518-6
Packager : Clinical Solutions Wholesale
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LISINOPRIL AND HYDROCHLOROTHIAZIDELISINOPRIL AND HYDROCHLOROTHIAZIDE TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LISINOPRIL AND HYDROCHLOROTHIAZIDELISINOPRIL AND HYDROCHLOROTHIAZIDE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LISINOPRIL AND HYDROCHLOROTHIAZIDELISINOPRIL AND HYDROCHLOROTHIAZIDE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
LISINOPRIL AND HYDROCHLOROTHIAZIDE TABLETS USP
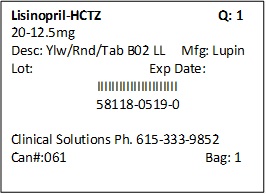
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL









