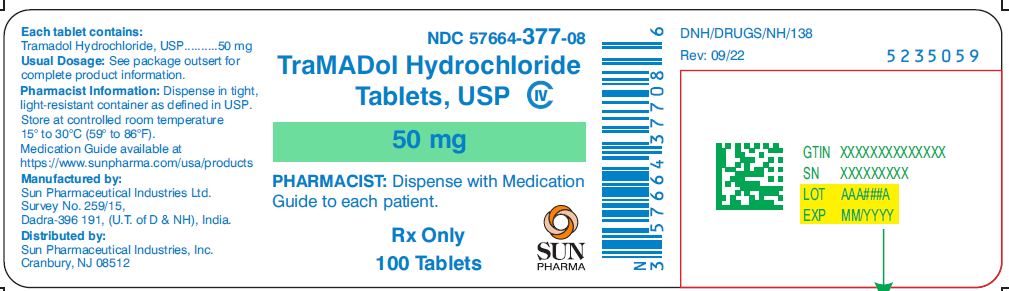
NDC Code(s) : 57664-377-08, 57664-377-13, 57664-377-18
Packager : Sun Pharmaceutical Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| tramadol hydrochloridetramadol hydrochloride TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries Limited | 650445203 | manufacture(57664-377) | |
PRINCIPAL DISPLAY PANEL
50mg-100 Tablets