NDC Code(s) : 52125-416-01
Packager : REMEDYREPACK INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lantusinsulin glargine INJECTION, SOLUTION | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
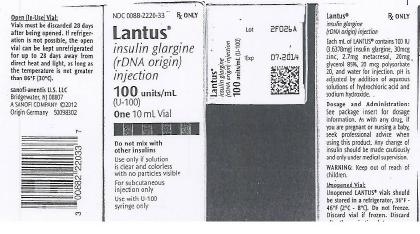
PRINCIPAL DISPLAY PANEL
DRUG: Lantus
GENERIC: insulin glargine
DOSAGE: INJECTION, SOLUTION
ADMINSTRATION: SUBCUTANEOUS
NDC: 52125-416-01
ACTIVE INGREDIENT(S):
- insulin glargine 100[iU] in 1mL
INACTIVE INGREDIENT(S):
- hydrochloric acid
- polysorbate 20
- water
- METACRESOL
- sodium hydroxide
- zinc
PACKAGING: 10 mL in 1 VIAL, GLASS

/>








