NDC Code(s) : 42023-142-01, 42023-143-25
Packager : Par Pharmaceutical, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Trimethobenzamide Hydrochloridetrimethobenzamide hydrochloride INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Trimethobenzamide Hydrochloridetrimethobenzamide hydrochloride INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 42023-142-01
Trimethobenzamide
Hydrochloride
Injection
2,000 mg/20 mL
(100 mg/mL)
NOT FOR USE IN CHILDREN
For Intramuscular (IM) Use Only
(preferably by deep IM injection)
20 mL x 1 Multiple Dose Vial

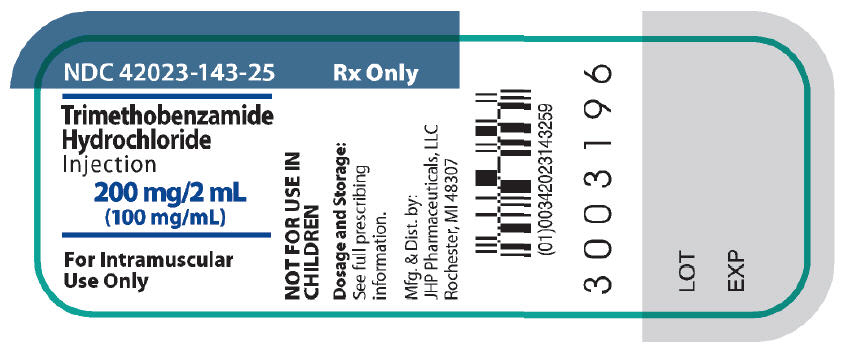
PRINCIPAL DISPLAY PANEL
NDC 42023-143-25
Trimethobenzamide
Hydrochloride
Injection
200 mg/2 mL
(100 mg/mL)
For Intramuscular
Use Only