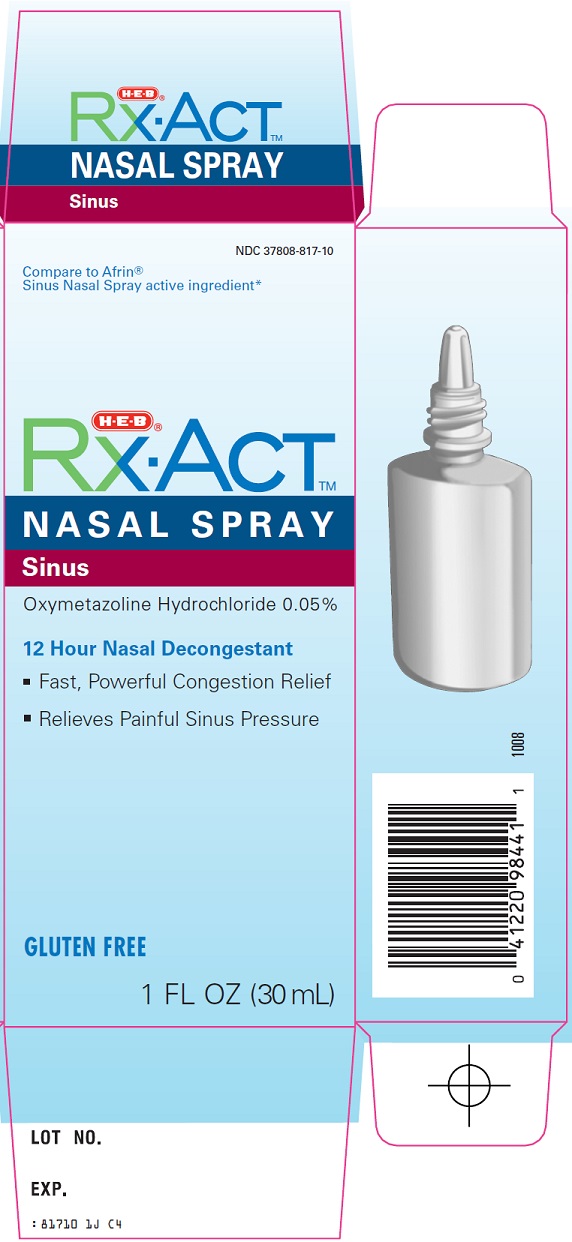
NDC Code(s) : 37808-817-10
Packager : H E B
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| rx act nasal oxymetazoline hydrochloride SPRAY | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Compare to Afrin® Sinus Nasal Spray active ingredient
NASAL SPRAY
Sinus
Oxymetazoline Hydrochloride 0.05%
12 Hour Nasal Decongestant
Fast, Powerful Congestion Relief
Relieves Painful Sinus Pressure
GLUTEN FREE
1 FL OZ (30 mL)