NDC Code(s) : 16729-277-30, 16729-277-03, 16729-277-35
Packager : Accord Healthcare, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MethotrexateMethotrexate INJECTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Accord Healthcare, Inc.(604222237) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Intas Pharmaceuticals Limited | 725927649 | manufacture(16729-277), analysis(16729-277) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Intas Pharmaceuticals Limited | 915837971 | manufacture(16729-277), analysis(16729-277) | |
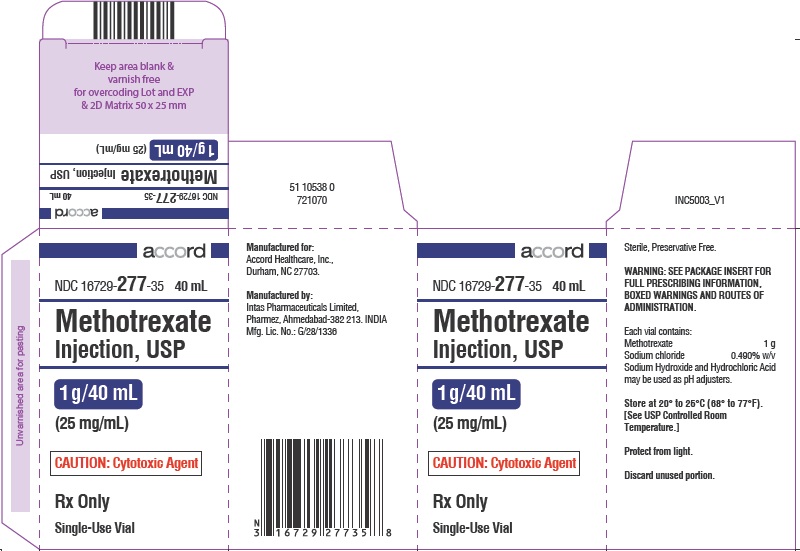
PRINCIPAL DISPLAY PANEL
NDC 16729-
277-30
2 mL
Methotrexate
Injection, USP
50 mg/2 mL (25 mg/ml)
CAUTION: Cytotoxic Agent
Rx Only Single-Use Vial

PRINCIPAL DISPLAY PANEL
NDC 16729-
277-30
2 mL
Methotrexate
Injection, USP
50 mg/2 mL
(25 mg/mL)
CAUTION: Cytotoxic Agent
Rx Only
Single-Use Vial
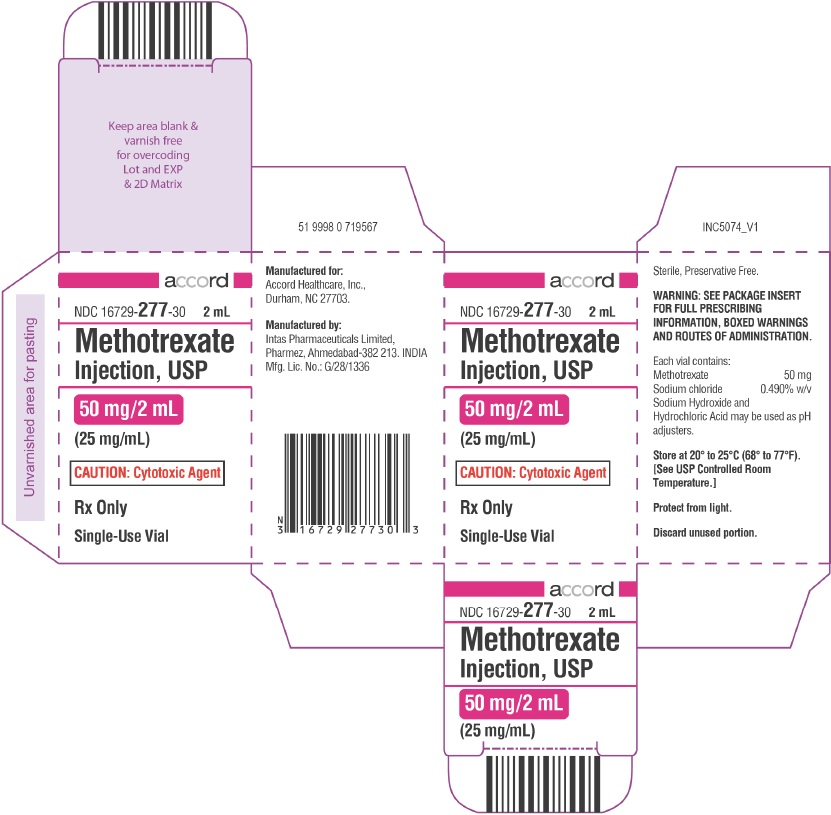
PRINCIPAL DISPLAY PANEL
NDC 16729-
277-03
10 mL
Methotrexate
Injection, USP
250 mg/10 mL
(25 mg/mL)
CAUTION: Cytotoxic Agent
Rx Only Single-Use Vial

PRINCIPAL DISPLAY PANEL
NDC 16729-
277-03
10 mL
Methotrexate
Injection, USP
250 mg/10 mL
(25 mg/mL)
CAUTION: Cytotoxic Agent
Rx Only
Single-Use Vial
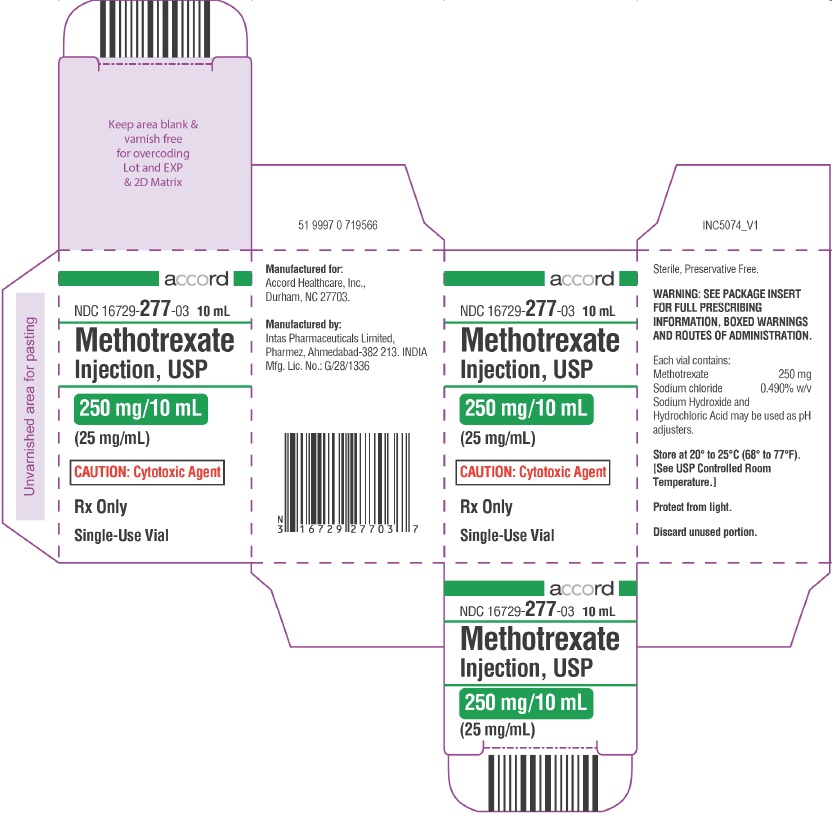
PRINCIPAL DISPLAY PANEL
NDC 16729-
277-35
40 mL
Methotrexate
Injection, USP
1 g/40 mL
(25 mg/mL)
CAUTION: Cytotoxic Agent
Rx Only Single-Use Vial

PRINCIPAL DISPLAY PANEL
NDC 16729-
277-35
40 mL
Methotrexate
Injection, USP
1 g/40 mL
(25 mg/mL)
CAUTION: Cytotoxic Agent
Rx Only
Single-Use Vial